Isolation and Characterization of Heterotrophic Nitrifying Strain W1*
2012-03-22Yongkang吕永康WANGXun王荀LIUBokai刘博凯LIUYuxiang刘玉香andYANGXiaohua杨晓华KeyLaboratoryofCoalScienceandTechnologyTaiyuanUniversityofTechnologyTaiyuan0004China
LÜ Yongkang (吕永康)**, WANG Xun (王荀) LIU Bokai (刘博凯), LIU Yuxiang (刘玉香) and YANG Xiaohua (杨晓华) Key Laboratory of Coal Science and Technology, Taiyuan University of Technology, Taiyuan 0004, China
2 Engineering & the Built Environment (EBE), University of Cape Town, Cape Town7701, South Africa
3 College of Environmental Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China
1 INTRODUCTION
Ammonium treatment is essential for preventing water eutrophication in aquatic environments. Wastewater, such as untreated coking wastewater, contains not only high concentration of nitrogen compounds but also high concentration of carbon materials. In untreated coking wastewater, ammonium may reach a concentration of 1000-3000 mg·L-1. It is not applicable to use conventional nitrification with autotrophic bacteria to treat such wastewater because of the following reasons. Firstly, the nitrification rate is extremely slow.To treat high concentration ammonium, large-scale device and long hydraulic retention time (HRT) are required [1-6]. Secondly, the autotrophs are vulnerable to high loads of ammonium and organic matter [7, 8].Thus, conventional nitrification can be implemented only after the wastewater is pretreated to reduce C/N ratio [9] or diluted [1, 10]. Thirdly, the system with aerobic/anaerobic conditions requires one aerobic tank and one anaerobic tank, demanding large space [9].
Compared with traditional autotrophic nitrification, heterotrophic nitrification presents larger growth rate, higher production of cells and higher adaptability to heterotrophic nitrifiers [11]. Recently, heterotrophic nitrifier has been intensively studied as potential microorganisms that may be used to overcome problems inherent in the conventional method. However, most of study has focused on low concentration ammonium discharged from domestic wastewater, with less attention on the treatment of high concentration ammonium in coking wastewater.
The objectives of this study are to isolate heterotrophic nitrifying bacteria from activated sludge of coking wastewater and examine the ability of the bacterium to remove ammonium from the medium containing high concentration ammonium.
2 MATERIALS AND METHOD
2.1 Isolation of bacterium
2.1.1Medium
Enrichment medium: beef extract-peptone medium [12].
Basic medium: (NH4)2SO42.0 g, K2HPO40.75 g,NaH2PO40.25 g, MgSO40.03 g, MnSO40.01 g, sodium citrate 5.0 g, H2O 1000 ml, pH=7.2 (According to Stephenson medium [13], calcium carbonate was replaced by sodium citrate in same mass in order to screen one strain of heterotrophic bacterium).
Improved medium: (NH4)2SO42.0 g, K2HPO40.75 g, NaH2PO40.25 g, MgSO40.03 g, MnSO40.01 g,sodium citrate 17.8054 g, H2O 1000 ml, pH=7.0.
2.1.2Enrichment
The beef extract-peptone medium was autoclaved at 121 °C for 30 min. Sterile medium (120 ml) in 250 ml conical flasks (n=3) was inoculated with 2 ml of fresh activated sludge from the coking wastewater,and incubated at 30 °C on a rotary shaker at 120 r·min-1.Every 3 days, 0.1 ml of medium was spot-tested for the consumption of ammonium using Nessler’s reagent [13].
2.1.3Isolation of ammonium oxidizing bacteria
When the Nessler’s reagent tests were proved positive, 1 ml of the enrichment cultures was transferred to basic medium. Some ammonium oxidizing bacteria were isolated by limited dilution method [14]. Purified isolate was obtained by repeated streaking on fresh washed-agar plates (liquid selective medium and 2% washed agar).
2.1.4Preliminary identification of strain performance
The isolated strain was tested for its ability to remove nitrogen. The strain was inoculated into 250 ml conical flasks (n=3) containing 120 ml of selected liquid medium and then cultured at 30 °C on a rotary shaker at 120 r·min-1. Every day, the medium samples were taken and analyzed for ammonia, nitrite, total nitrogen, mass growth and change in pH. This operation was repeated once every day for 7 days.
2.2 Identification of the isolated strain
Physiological and biochemical characteristics of the isolated bacterium were examined according to the standard methods described previously [15]. The 16S rRNA sequence of the isolate was tested by TaKaRa Biotechnology (Dalian) Co., Ltd. The isolated strain was compared with other microorganisms using the Basic Local Alignment Search Tool program, to decide its species.
2.3 Optimization of basic medium
2.3.1Carbon sources
The isolated strain was cultured in the basic medium with the substitution of sodium citrate by different carbon sources at the same moles of carbon. The medium containing glucose was autoclaved at 110 °C for 15 min, and all other media at 121 °C for 20 min.The amount of (NH4)2SO4(N source) was fixed, providing 423.0-424.0 mg·L-1NH4-N. The bacterium was incubated at 30°C under aerobic condition on a rotary shaker at 120 r·min-1for 4 days. Then a basic medium with the highest nitrogen removal efficiency was obtained. The carbon source contained in the selected medium was regarded as the optimal carbon source.
2.3.2Nitrogen sources
With the optimal carbon source, isolated strain was cultured in the basic media with the replacement(equivalent moles of nitrogen) of ammonium sulfate by different nitrogen sources. All media were autoclaved at 121 °C for 20 min. The culture conditions were as above. After incubation for 4 days, a basic medium with the highest nitrogen removal efficiency was obtained. The nitrogen source contained in the selected medium was regarded as the optimal nitrogen source.
2.3.3C/N ratio
With the optimal organic carbon and nitrogen source, the influence of C/N ratio in the selected medium on ammonium removal by isolated strain was investigated. The C/N ratio was adjusted to 2, 4, 6, 8,10, 12, 14, 16, 20 and 30 separately by fixing the amount of (NH4)2SO4(optimal nitrogen source) and adding sodium citrate (optimal carbon source). The culture conditions were as same as mentioned above.After incubation for 4 days, a basic medium with the highest nitrogen removal efficiency was obtained. This C/N ratio of the medium was regarded as the optimal C/N ratio.
2.3.4Initial pH of selective medium
With the optimal carbon source, nitrogen source and C/N ratio, the effects of initial pH in basic media on ammonium removal by strain W1 were studied.Initial pH of medium was adjusted to 4, 5, 6, 7, 8, 9 and 10 separately by adding 0.1 mol·L-1NaOH or 0.1 mol·L-1HCl solution. The culture conditions were as same as mentioned above. The comparisons of the ammonium removal in different media (different initial pH) gave the optimum initial pH of medium for the bacterium.
2.4 Comparative study on nitrification and growth of strain W1 in basic and improved media
One milliliter of cell in logarithmic phase was inoculated in 100 ml of optimum medium and selected medium separately. The bacterium was cultivated for 4 days. The culture conditions were as above. The culture media in the improved medium and basic medium were sampled for chemical analyses.
2.5 The influence of initial ammonium concentration
With the optimal medium, the influence of ammonium concentration on the nitrifying capability of strain W1 was measured. Initial NH4-N concentration was separately adjusted to 50, 100, 200, 400, 600, 800,1000, 1200, 1500, 2000 and 3000 mg·L-1using ammonium sulfate and sodium citrate by fixing C/N ratio and initial pH. The culture conditions were as same as mentioned above.
2.6 Identification of aerobic denitrification in pure oxygen system
In order to identify whether isolated strain has heterotrophic nitrification and aerobic denitrification abilities simultaneously, the production of N2gas by isolated strain incubated for 96 h in the improved medium under pure oxygen system was examined. The culture conditions were as above. In the test, 2 ml of the bacterium was inoculated in a 1 L flask containing 200 ml improved medium. Then the sealed flask was filled with pure oxygen in clean environment. To ensure that the system was not infected by other bacteria,air bacterial filter was used during the infusing oxygen process. The equipment was shown in Fig. 1. At the beginning and end of the test, the gas samples in the medium were analyzed using gas chromatography [16].
2.7 Analytical methods

Figure 1 Chart of 1L airproof-flask with two oraes1—biforate rubber stopper; 2—gas sampling point; 3—valve;4—air inlet port (rubber tube with clamp was connected with glass tube); 5—air outlet port (rubber tube with clamp was connected with glass tube)
Growth of the bacterium was monitored by measuring the optical density (OD600) using a spectrophotometer. The concentrations of ammonium (4NH+-N)and nitrite (2NO--N) were analyzed using Nessler’s reagent photometry andN-(1-naphthalene)- diaminoethane photometry, respectively. Concentrations of nitrate (3NO--N), total nitrogen (TN) and total carbon(TC) were measured by ion chromatography and TOC-TN analyzer. N2and O2were assayed by gas chromatography.
3 RESULTS AND DISCUSSION
3.1 Identification of strain W1
One heterotrophic bacterium with high-strength ammonium removal ratio and total nitrogen removal ratio was isolated, and named as W1. Strain W1 is a nonmotile, Gram-negative, short rod bacterium, having no endospore. It grows as yellow, opaque, round colonies on plate of solid basic medium. In a series of physiological and biochemical tests, strain W1 is positive for catalase reaction, gelatin liquefaction, starch hydrolysis and oxidase reaction, and negative for V-P test, M-R test,sugar fermentation and indole test. With 16S rRNA obtained by PCR amplification, we observe 99% sequence similarity with that ofAlcaligenessp. F78(GenBank accession number EU443097.1). On the basis of the above results, strain W1 is confirmed primarily asAlcaligenessp., named asAlcaligenessp. W1.
3.2 Preliminary investigation of strain performance

Figure 2 Growth and ammonium removal efficiency of strain W1 in selective medium
Figure 2 shows the growth in 50 h and ammonium and nitrite concentrations in 7 days when strain W1 was cultivated in basic medium under aerobic condition. The lag phase of strain W1 was only 6 h,indicating that strain W1 has excellent adaptability to the medium containing high concentration ammonium.After 6 h, OD600value increased rapidly. The strain reproduced rapidly. The maximum growth rate was between 16 h and 36 h, with a maximum growth rate of 0.5526 h-1. However, strain W1 accessed to decline phase after 36 h incubation without stationary phase,implying that no adequate nutrition was provided for growth in the basic medium after 36 h incubation,possibly lacking of carbon source. In the prime of culture,4NH+-N, the sole N-source, decreased dramatically.The maximum removal rate of4NH+-N was 121.10 mg·L-1·d-1.4NH+-N decreased from 434.47 mg·L-1to 64.75 mg·L-1in 4 days. These data indicates that strain W1 has higher heterotrophic nitrogen removal ability in high concentration ammonium medium, compared withBrevibacillussp. LY (NH4-Nconcentration decreased from 45.30 mg·L-1to 9.89 mg·L-1in 24 days)[14, 17]. After 4 days, the residual content of NH4-N altered little. Thus, the cultural time was set to 4 days in the following experiments. The comparison of growth curve and ammonium removal curve indicates that the maximum ammonium removal rate appears in decline phase of growth, due to the nitrification, which is consistent with previous reports [18, 19].
3.3 Optimization of basic medium
3.3.1Optimization of carbon source
Related reports have shown that the ammonium removal ability of heterotrophic bacterium is strongly influenced by carbon sources. Table 1 showed the growth and nitrification ability of strain W1 in the media containing different carbon sources. The OD600values show that strain W1 grows better in the medium with citrate sodium, ethanamide, peptone and acetate sodium used as sole carbon source. Strain W1 utilizes organic carbon as well as inorganic carbon,indicating that strain W1 has broad adaptability to different organic matters. The comparison of initial and final4NH+-N contents shows different ammonium removal ability of strain W1 in different carbon sources, probably due to different metabolic pathways.Strain W1 has the best ammonium removal ability in the medium containing citrate sodium. Thus, citrate sodium was used as the optional carbon source for strain W1 in the following experiments.
3.3.2Optimization of nitrogen source
As shown in Table 2, strain W1 is adaptable to different nitrogen sources. It utilizes not only inorganic nitrogen sources, but also organic nitrogen sources. It means the ability of the strain to degrade

Table 1 The ammonium removal ability of strain W1 with different carbon sources

Table 2 The ammonium removal ability of strain W1 with different nitrogen sources
3.3.3C/N ratio

Figure 3 Nitrification characteristic and growth of strain W1 at different C/N ratios1—final 4NH+-N; 2—final TN; 3—initial 4NH+-N; 4—initial TN; 5—final 2NO--N; 6—OD600
C/N ratio is one of the main important factors for heterotrophic nitrification bacteria. In this study, the ammonium removal of strain W1 in high-strength ammonium medium was investigated by changing C/N ratio (Fig. 3). OD600value increased almost linearly with the C/N ratio up to 8, but further increment of C/N ratio did not affect the growth of strain W1. It indicates that the growth of bacterium is affected by C/N ratio when the carbon source is insufficient, but strain W1 presents excellent adaptability to high concentration organic matter. Owing to its good adaptability to different C/N ratios, strain W1 would have a bright foreground of application in coking wastewater treatment. As shown in Fig. 3, the final4NH+-N concentration first decreases and then increases with the increase of C/N ratio, as does the final TN value. The final concentration of4NH+-N is the maximum at C/N 0.5 (377.64 mg·L-1) and the minimum at C/N 12(28.24 mg·L-1), indicating that the ammonium removal ability of strain W1 is influenced significantly by C/N ratio. At C/N=12, the removal efficiency of-N is higher. Thus, the optimum C/N ratio is 12.
Furthermore, the ammonium removal ability and the growth of strain W1 at high (C/N=20), intermediate (C/N=12) and low (C/N=5) C/N ratios were investigated. Figs. 4 and 5 present the nitrification process and growth of bacterium at different C/N ratios,respectively. In Fig. 4, at C/N=12 and C/N=20, the final concentration of4NH+-N is about 25 mg·L-1,which meets the national discharge standard of class II,while at C/N=5, it is above 100 mg·L-1, mainly because the exhaustion of carbon source affects the removal ability and growth of bacterium. After cultivation of 84 h, the concentrations of4NH+-N and TN are lower at C/N=12, indicating that C/N=12 is the best C/N ratio for strain W1. The4NH+-N and TN removal ratio were up to 98.32% and 78.38%, respectively, at C/N=12 after 84 h cultivation (data not shown). In Fig. 5, logarithmic growth phase of strain W1 has no remarkable difference at C/N of 5, 12 and 20, suggesting that C/N ratio has little influence on reproduction rate of strain. It is inferred that, in industrial applications, the C/N ratio should be strictly controlled in ammonium removal phase, but it is not needed in the cultivation phase.

Figure 4 The nitrification of strain W11—TN(C/N=5); 2—TN(C/N=12); 3—TN(C/N=20);4—- N(C/N=5); 5—-N(C/N=12);6—-N(C/N=20); 7—-N(C/N=5);8—-N(C/N=12); 9—-N(C/N=20)

Figure 5 The growth of strainW11—OD600(C/N=5); 2—OD600(C/N=12); 3—OD600(C/N=20)
Figure 6 gives the4NH+-N and TC concentrations in selected medium at C/N 12. The two concentrations decrease with time in parallel, indicating that the carbon and nitrogen sources are utilized at a well-balanced ratio. This testifies that C/N 12 is the best condition for strain W1. In 84 h, ammonium concentration decreases to 28.04 mg·L-1, and TC concentration is 3242.50 mg·L-1. The remained TC could be further used as carbon source for denitrification. Thus,strain W1 exhibits remarkable nitrification performance at C/N 12. Other characteristics of strain W1 were investigated at C/N ratio of 12.
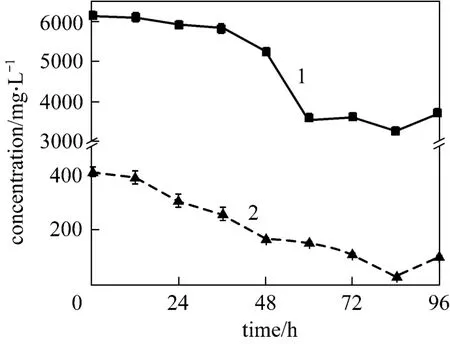
Figure 6 -N and TC concentrations vs. time 1—TC; 2—-N
3.3.4Initial pH
In order to examine the adaptability of strain W1 to environment, the ammonium removal of strain W1 at different initial pH (4-10) after 4 days cultivation was investigated. Fig. 7 shows the ammonium removal and the growth of strain W1 at different initial pH in the basic medium with C/N ratio of 12. The curves can be divided into three stages: pH=4-5,pH=6-9, and pH=10.

Figure 7 Ammonium removal and growth of strain W1 at different initial pH1—initial 4NH+-N; 2—final TN; 3—final 4NH+-N; 4—OD600
At the initial pH values of 4-5, strain W1 does not grow in the basic medium, and therefore does not remove ammonium, because the bacterium is dead under strong acid condition. At the initial pH values of 6-9, strain W1 grows well (OD600≥2.0), indicating that strain W1 has good adaptability to the environment. Meanwhile strain W1 presents favorable ammonium removal ability (final4NH+-N<100 mg·L-1).Thus strain W1 may be further used in industrial applications, especially for coking wastewater treatment.At pH 7, the NH4-N concentration is the lowest and the OD600value is the largest for pH 6-9. The ammonium removal is up to 97.09%. Thus pH 7 is the best pH value for strain W1, close to the optimum range for nitrifying bacteria [20]. At the initial pH value of 10, strain W1 does not grow in the basic medium, but part of ammonium in the medium is removed. This is possibly because the removed ammonium leaves the system, which is strong alkaline environment, in the form of ammonia.
3.4 Comparative study on nitrification of strain W1 in basic and improved media
Figure 8 shows the nitrification ability of strain W1 in the basic and improved media after 4 days cul-tivation. The final -N and TN concentrations are lower in the improved medium. The removal ratio of -N and TN are improved remarkably, from 78.49% and 55.92% to 91.98% and 79.02%, respectively. Therefore, strain W1 exhibits better nitrification ability in the improved medium. Optimization of medium is a highly effective method for enhancing the removal efficiency of ammonium.2NO--N accumulation is also lower in the improved medium, which is consistent with the report by Glasset al. that the accumulation of the nitrite intermediate was associated with the inhibition of denitrification [21].

Figure 8 Comparison of nitrification ability in basic medium and improved mediaimproved;basic;blank control
3.5 Influence of ammonium concentration

Figure 9 The growth and nitrification of strain W1 in media with different substrate concentrations1—removal of 4NH+-N; 2—removal of TN; 3—OD600
In industrial productions, substrate concentration is not constant. It is necessary to examine the adaptability of bacterium to different substrate concentrations. Fig. 9 shows the influence of ammonium concentration on the growth and ammonium removal of strain W1 in the improved medium. The optical density value first increases and then decreases with the increase of initial ammonium concentration (IAC) in the media. At the IAC below 2000 mg·L-1, strain W1 presents excellent growing state in the media, though at the IAC of 3000 mg·L-1, strain W1 grows poorly.Thus strain W1 exhibits excellent substrate tolerance in the media containing high substrate concentrations.However, the ammonium removal ability of strain W1 presents somewhat different trend. At the IAC below 1200 mg·L-1, strain W1 shows good removal rate of-N and TN, especially at 200 mg·L,-N and TN removal reaches their maximum values,-192.34% and 81.41%, respectively. When IAC ranges from 1200 to 2000 mg·L-1, strain W1 presents good growth but bad ammonium removal ability, mainly due to the prolonged adaptable phase with the increase of substrate concentration. In coking wastewater treatment, if the hydraulic retention time (HRT) of biological treatment is prolonged appropriately, it is possible to omit the pretreatment of coking wastewater and access directly to biological treatment. If so, the cost of wastewater treatment will be reduced substantially. Compared with strain A1[22], which was not able to efficiently transform the substrate at concentrations higher than (536.21±3.15) mg·L-1, strain W1 presents excellent adaptability and good nitrification ability in the media containing high ammonium concentrations.
To further explain the trend about the growth and ammonium removal of strain W1 at different substrate concentrations, the growth of strain in the medium containing low (100 mg·L-1), intermediate (400 mg·L-1) and high (1200 mg·L-1)4NH+-N concentrations was investigated. As shown in Fig. 10, the adaptable growth phase of strain W1 prolongs as INC increases, but at different INC, the growth rates of strain in logarithmic growth phase are of similar size.This indicates that the increment of INC has no influence on the activity of strain W1. Meanwhile, the ammonium removal rates increase with the increment of INC. They are 3.81, 7.05 and 12.95 mg·L-1·h-1in the media containing 100, 400 and 1200 mg·L-1ammonium, respectively. These data show that nitrificationability of strain W1 is not affected by the high substrate concentrations (INC≤2000 mg·L-1). Figs. 9 and 10 indicate the reason why the removal efficiency of ammonium is inversely proportional to INC. As the INC increases, the adaptation phase extends, so that the time used for nitrification is shorter, lowering the removal efficiency of ammonium. If the time is enough for nitrification for high concentration ammonium, strain W1 will have the same removal efficiency as in low-strength ammonium. Thus, strain W1 exhibits high activity and excellent adaptability to environment containing high COD and ammonium, which is suitable for potential industrial applications.

Table 3 The changes of gas phase compositions in closed system (%, by volume)

Figure 10 Growth and ammonium removal in three media containing different initial ammonium concentration1—final -N, with initial -N of 1200 mg⋅L-1; 2—final -N, with initial -N of 400 mg·L-1; 3—final-N, with initial -N of 100 mg⋅L-1; 4—OD600, with initial -N of 100 mg⋅L-1; 5—OD600, with initial -N of 400 mg·L-1; 6—OD600, with initial -N of 1200 mg⋅L-1
3.6 Preliminary identification of aerobic denitrification
It is known that the amount of intracellular nitrogen does not generally exceed 30% of total nitrogen.However, the TN removal in this study was up to 79.02% (Fig. 8). For the amount of intracellular nitrogen equal to 30% of total nitrogen, according to the nitrogen balance, 49.02% of TN was missing during the nitrogen removal. Hence, it is inferred that strain W1 performs aerobic denitrification besides heterotrophic nitrification. In order to identify the aerobic denitrification, the production of N2gas in a closed system was examined. The results shown in Table 3 testify that strain W1 has the ability of aerobic denitrification. At the same time, the removal efficiency of ammonium was 92.43% (data not shown). Thus, it can be concluded that strain W1 performs heterotrophic nitrification and aerobic denitrification simultaneously.The aerobic denitrification of strain W1 will be further studied.
4 CONCLUSIONS
Using limited dilution method, one nitrifying bacterial strain was isolated and named as W1. By a series of physiological and biochemical methods and 16S rRNA, strain W1 was identified asAlcaligenessp.The strain grew well and presented high ammonium removal ability in the medium containing high ammonium concentration (400 mg·L-1), and had excellent tolerance to high substrate concentration (INC≤2000 mg·L-1), with its activity affected little.
In the optimized medium, the growth and ammonium removal ability of strain W1 were obviously improved in 4-day cultivation, in which C/N ratio was the most important factor for strain W1 to improve the process. TN removal ratio reached around 80%. The removed nitrogen far exceeded the quantity of nitrogen used in synthesizing cell.
The production of N2gas in pure oxygen system was examined. The result suggests that strain W1 performs heterotrophic nitrification and aerobic denitrification simultaneously.
1 Rostron, W.M., Stuckey, D.C., Young, A.A., “Nitrification of high strength ammonia wastewater: comparative study of immobilization media”,Water Res., 35, 1169-1178 (2001).
2 Ilies, P., Mavinic, D.S., “The effect of decreased ambient temperature on the biological nitrification and denitrification of high ammonia landfill leachate”,Water Res., 35, 2065-2072 (2001).
3 Itokawa, H., Hanaki, K., Matsuo, T., “Nitrous oxide production in high-loading biological nitrogen removal process under low COD/N ratio condition”.Water Res., 35, 657-664 (2001).
4 Ruiz, G., Jeison, D., Chamy, R., “Nitrification with high nitrite accumulation for the treatment of wastewater with high ammonia concentration”,Water Res., 37, 1371-1377 (2003).
5 Carrera, J., Torrijos, M., Baeza, J.A., Lafuente, J., Vicent, T., “Inhibition of nitrification by fluoride in high strength ammonium wastewater in activated sludge”,Process Biochem., 39, 73-79(2003).
6 Schmidt, I., Sliekers, O., Schmid, M., Bock, E., Fuerst, J., Kuenen,J.G., Jetten, M.S.M., Strous, M., “New concepts of microbial treatment processes for the nitrogen removal in wastewater”,FEMS Microbiol.Rev., 772, 1-12 (2003).
7 Kim, D.J., Lee, D.I., Keller, J., “Effect of temperature and free ammonia on nitrification and nitrite accumulation in landfill leachate and analysis of its nitrifying bacterial community by fish”,Bioresour.Technol., 97, 459-468 (2006).
8 Kulikowska, D., Józ ´wiak, T., Kowal, P., Ciesielski, S., “Municipal landfill leachate nitrification in RBC biofilm-process efficiency and molecular analysis of microbial structure”,Bioresour.Technol., 101,3400-3405 (2010).
9 Khin, T., Annachhatre, A.P., “Novel microbial nitrogen removal processes”,Biotechnol.Adv., 22, 519-532 (2004).
10 Jung, J.Y., Chung, Y.C., Shin, H.S., Son, D.H., “Enhanced ammonia nitrogen removal using consistent biological regeneration and ammonium exchange of zeolite in modified SBR process”,Water Res.,38, 347-354 (2004).
11 Brierly, E.D.R., Wood, M., “Heterotrophic nitrification in an acid forest soil: Isolation and characterization of a nitrifying bacterium”,Soil Biol.Biochem., 33, 1403-1409 (2001).
12 Su, J.F., Ma, F., Wang, H.Y., Wei, L., “Isolation of heterotrophic nitrification bacteria and nitrifying character of bioceramic reactor”,Huazhong Univ.of Sci.& Tech., 35 (12), 125-128 (2007). (in Chinese)
13 Li, Z.G., Luo, Y.M., Teng, Y., Soil and Environmental Microorganisms Study, Science Press, Beijing (2008). (in Chinese)
14 Chen, P.Z., Wang, L.G., Wang, Y.C., Li, J., Ding, W., Ren, T.Z., Li,S.P., “Screening and Denitrification characteristics of a heterotrophic nitrification-aerobic denitrifier bacteria”,Environmental Science, 30(12), 3614-3618 (2009). (in Chinese)
15 MacFaddin, J.F., Biochemical Tests for Identification of Medical Bacteria, Williams & Wilkins, Baltimore, MD, 36-308 (1984)
16 Yin, M.R.,Wang, P., Liao, X.H.,Zhang, Z.J., Ma, J.F., Yu, Q.,“Experimental study on N2O and N2—products of ammonium removal by heterotrophic nitrification-aerobic denitrification strain WXZ-8”,Environmental Science and Technology, 33 (8), 16-23(2010). (in Chinese)
17 Lin, Y., Kong, H.N., He, Y.L., Yan, L., Li, C.J., “Isolation and characterization of heterotrophic nitrifying bacteria”.Environmental Science, 27 (2), 324-328 (2006).
18 Zhang, G.Y., Chen, M.C., Han, R.Y., Min, H., “Isolation, identification and phylogenetic analysis of a heterotrophic nitrifier”,Microbiologica.Sinica, 43 (2), 156-161 (2003).
19 Verstrate, W, Alexander, M., “Heterotrophic nitrification byArthrobactersp.”,Bacteriol., 110, 955-961 (1972).
20 Antoniou, P., Hamilton, J., Koopman, B., Jain, R., Holloway, B.,Lyberatos, G., Svoronos, S.A., “Effect of temperature and pH on the effective maximum specific growth rate of nitrifying bacteria”,Water Research, 24, 97-101 (1990j).
21 Glass, C., Silverstein, J., Oh, J., “Inhibition of denitrification in activated sludge by nitrite”,Wat.Environ.Res., 69, 1087-1093 (1997).22 Yang, X.P., Wang, S.M., Zhang, D.W., Zhou, L.X., “Isolation and nitrogen removal characteristics of an aerobic heterotrophic nitrifying-denitrifying bacterium,Bacillus subtilisA1”,Bioresource Technology, 102, 854-862 (2011). (in Chinese)
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- The Short-term Effects of Temperature and Free Ammonia onAmmonium Oxidization in Granular and Floccular Nitrifying System*
- Adsorption and Desorption of Praseodymium (III) from Aqueous Solution Using D72 Resin*
- Ammoximation of Cyclohexanone to Cyclohexanone Oxime Catalyzed by Titanium Silicalite-1 Zeolite in Three-phase System*
- Turbulent Characteristic of Liquid Around a Chain of Bubbles in Non-Newtonian Fluid*
- Recovery of Tungsten (VI) from Aqueous Solutions by Complexationultrafiltration Process with the Help of Polyquaternium*
- Optimizing the Chemical Compositions of Protective Agents for Freeze-drying Bifidobacterium longum BIOMA 5920*
