Venting Design for Di-tert-butyl Peroxide Runaway Reaction Based on Accelerating Rate Calorimeter Test
2012-02-14WEITongtong魏彤彤andJIANGHuiling蒋慧灵
WEI Tongtong (魏彤彤)* and JIANG Huiling (蒋慧灵)
Department of Fire Protection Engineering, Chinese People’s Armed Police Force Academy, Langfang 065000, China
1 INTRODUCTION
Di-tert-butyl peroxide (DTBP) is widely used as reaction initiator and cross-linking agent in high polymer synthesis industry, but its thermal instability and oxidizability makes it very easy to decompose and release large volume of gas, which may lead to explosion accident because of high pressure in the storage and transport tanks. Thus it is necessary to design an appropriate emergency relief system for DTBP tanks.
In China, the criterions of emergency relief systems for reactive liquids are not perfect [1]. In this study, Design Institute for Emergency Relief System(DIERS) method is introduced to calculate the relief system size. The accelerating rate calorimeter (ARC)is used, which is highly adiabatic, allows a small amount of testing sample, and the results can be extrapolated to any size. Thermal and pressure hazard parameters can be derived from the data, including onset temperature, adiabatic temperature rise, pressure generation rate, time needed to reach the maximum rate, and temperature of no return. The thermodynamic and kinetic information for runaway reactions can be acquired to investigate the cause of explosion and provide the most serious conditions for relief system size design [2].
2 EXPERIMENTAL
The apparatus is provided by Department of Fire Protection Engineering of Chinese People’s Armed Police Forces Academy. A typical test employs a titanium bomb loaded under 101.325 kPa (1 atm). The bomb is kept in a nearly perfect adiabatic environment,and the sample is heated by a fixed increment of temperature, searching for exothermic reaction and checking if the self-heating rate of sample exceeds the slope sensitivity. Once an exothermic reaction is detected, the data of time, temperature, self-heating rate and pressure are collected until the reaction finishes.
The sample DTBP is in chemical pure. The mass of samplemfis 1.1766 g. Its specific heatcfis 2.1 kJ·kg-1·K-1. The volume of sample bombVeis 8.75 cm3. Its massmbis 8.8133 g and its specific heatcbis 0.523 kJ·kg-1·K-1. Thermal inertia factorφ=2.865,which is calculated by Eq. (1) [3].
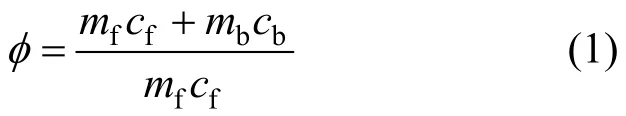
3 TESTING RESULTS AND MODIFICATION OF DATA
The test results of DTBP by ARC are shown in Figs. 1-4 and the second column in Table 1.
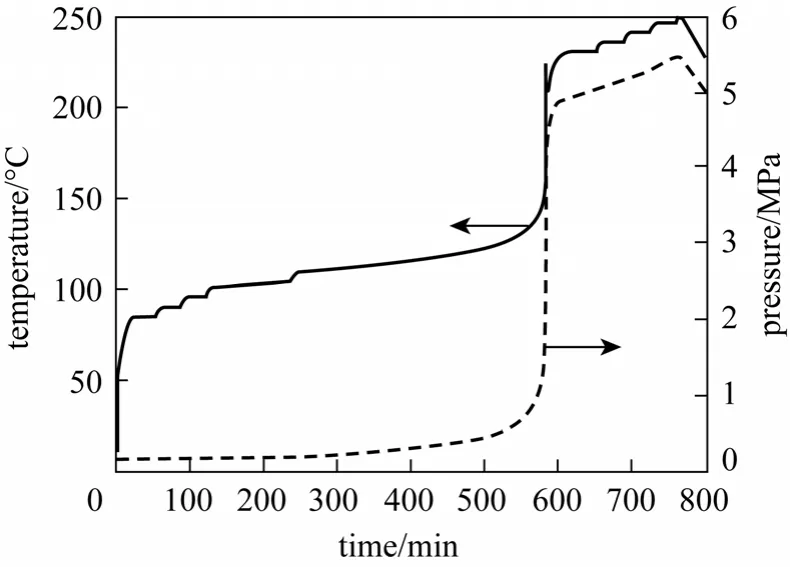
Figure 1 Temperature and pressure vs. time
3.1 Adiabatic modification
Because the test system is not absolutely adiabatic,in order to investigate the explosion under the worst condition, it is necessary to modify the tested data with thermal inertia factor. The kinetic parameters such as apparent activation energyEaand pre-exponential factorA*of the thermal decomposition are calculated by pseudo-inverse matrix method with the results ofn=1,Ea=169.68 kJ·mol-1, andA*=3.34×1019[4].
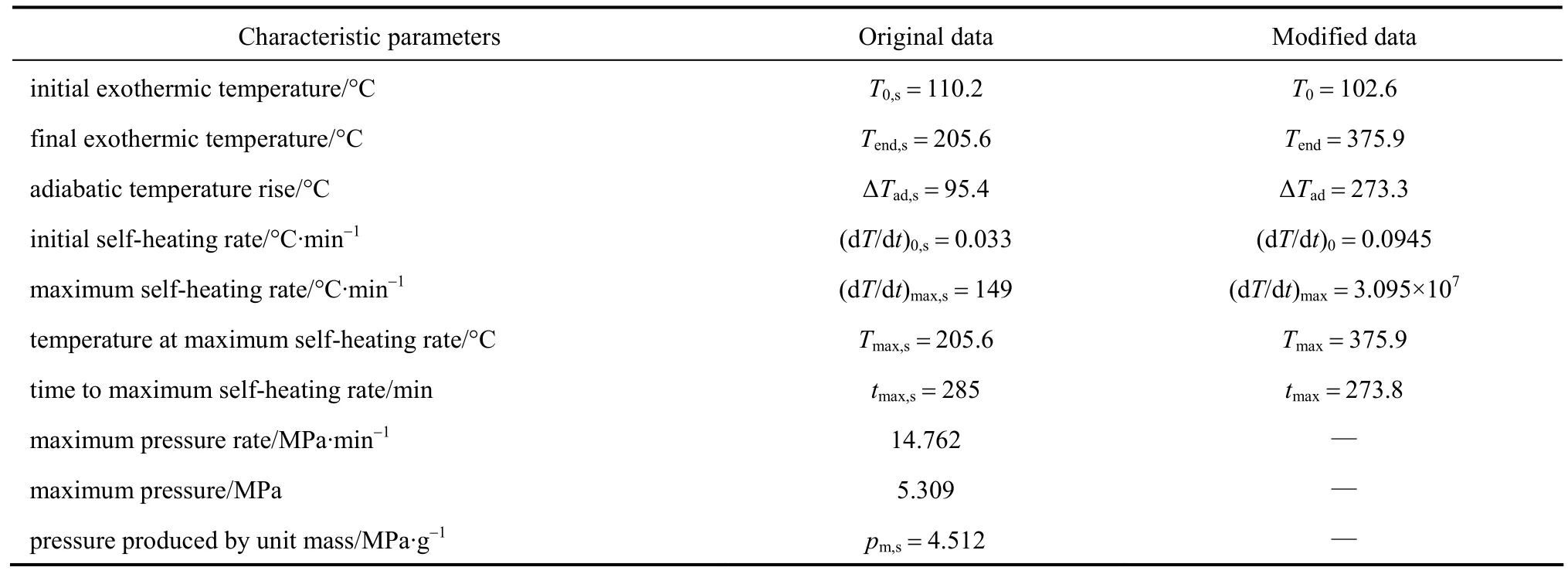
Table 1 Measured and modified thermal decomposition characteristic data of DTBP (φ=2.865)

Figure 2 Rise rates of pressure and temperature vs. temperature during exothermal stage

Figure 4 Curves of temperature vs. pressure

Figure 3 Exothermal curve of temperature vs. pressure
Then the adiabatic temperature rise ΔTad, the initial temperatureT0, the final temperature Tend and the time needed to reach the maximum self-heating ratetmaxcan be modified by thermal inertia factor from Eqs. (2)-(7) [5], as shown in the third column of Table 1.
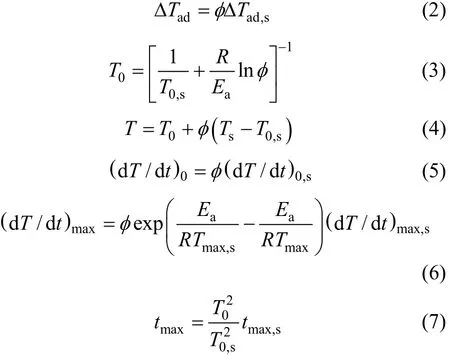
whereRis the gas constant, subscript s refers to the reaction parameters including sample and sample bomb.
From Table 1, it can be seen that under adiabatic condition, the onset exothermic temperature is 102.6 °C and after 285 min, the reaction is the most drastic with the maximum self-heating rate of 3.095×107°C·min-1,and the reaction reaches the maximum temperature 375.9 °C, which is also the final temperature. The adiabatic temperature rise, final temperature, the maximum self-heat rate and maximum self heat rate temperature are higher under adiabatic condition than those under test condition, but the onset exothermic temperature is lower. The lower the thermal inertiaφ,the more easily the runaway reaction will take place.If reactant is excessive or the released heat cannot disperse in time, the reaction will be runaway and the emergency relief system should start, otherwise fire or explosion accident will take place. In order to reduce the damage from runaway reaction, the venting size should be suitable for emergency relief.
3.2 Pressure mapping
Pressure mapping is necessary for two reasons.Firstly, whether a vessel is in the relief state depends on the working pressure. If the working pressure reaches the designed relief pressure, the relief device will work, so parameters (temperature, self-heating rate, pressure rising rate) at relief state are crucial to the design of relief size. Secondly, because of the difference between experimental and industrial storage,the thermodynamic parameters (such as temperature and self-heating rate) under industrial conditions are much different from experimental data even at the same pressure. One way to solve these problems is to find a relationship between the experimental pressure and the designed relief pressure as in Eqs. (8) and (9) [6].
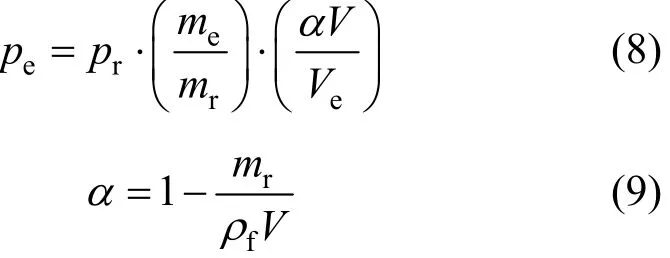
wherepis the pressure, MPa;mis the mass, kg;ρfis the liquid density, kg·m-3;Vis the volume, m3;αis the void fraction, subscriptsrand e refer to industry condition and test condition, respectively.
A storage tank of 5 m3loaded with 2000 kg DTBP has a design pressurepr=2.2 MPa and a maximum accumulated pressurepm=3.2 MPa. From Eq. (8),pr=0.37 MPa andpm=0.54 MPa under ARC experimental conditions (α=0.497). The modified physical parameters of DTBP are listed in Table 2.
4 RELIEF SYSTEM DESIGN
4.1 Classification of relief system
The reacting system may be vapor, gas or hybrid(tempered and non-tempered), depending on which fluid has dominant contribution to pressure rising. The type of reacting system can be determined by ARC tests. ARC test is carried out until the end of exothermal reaction and then cooled with all data recorded.By analyzing lgpvs. (1/T) curve, if the pressure falls to the original value due to cooling of compressible gases, it reveals a vapor system. If the pressure shows little reduction due to cooling of incompressible gases,it reveals a gassy system. If the pressure reduces to intermediate between the two systems, it reveals a hybrid system. When the pressure of vapor is lower than 10% of the total pressure, the system is non-tempered, otherwise it is tempered [7].
The DTBP reacting system is a hybrid system. In the ARC test, the vapor pressure is in direct proportion to vapor generating rateQVand gas pressure is in direct proportion to gas generating rateQG.QVandQGcan be calculated by Eqs. (10) and (11) [8]. Since
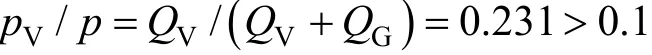
the system is tempered.

4.2 Calculation of relief system size
Wcan be calculated by Leung method as shown in Eq. (12) [9, 10], whereqis the average heat release rate per unit mass of reacting mixture, depending on the absolute overpressurer. ΔtBin Eq.(13) is called Boyle time which means the time taken for the pressure to rise from the relief pressure to the maximum accumulated pressure. In this emergency relief circumstance, the absolute overpressure is 0.31,so=343.3 W·kg-1. ΔTHis the temperature change asthe pressure rises frompmtoprand the detailed calculation equations can be found in reference [11]. The final calculation result forWis 198.7 kg·s-1.

Table 2 Physical property of DTBP under experimental conditions

Gcan be obtained by omega (ω) method, which includes a series of simplification assumptions but is convenient to apply because it does not require a complex computer code for its evaluation andωis a dimensionless parameter criterion for compressibility of two-phase mixture, whose definition equation can be found in reference [12]. Whenω<1, it is a non-flashing two-phase system; whenω=1, it is an incompressible gas single-phase system; whenω>1, it is a flashing two-phase system. In the omega method,the calculation ofGfor hybrid system consists of two main steps: obtain the mass velocityGGfor gas relief system assuming that the system consists of non-flashing liquid and permanent gas, and obtain the mass flow velocityGVfor vapor system assuming that the system consists of only flashing liquid.Gcan be calculated as follows.

whereyVis the mole fraction of vapor in the gas/vapor phase under stagnation condition at the inlet to the relief line,yV=pV/p.
Based onωmethod, there are two calculation methods forGGandGV, which are monograph and arithmetic calculation. In the monograph method,three main correction factors (dimensionless mass flux, friction correction factor, and back pressure correction factor) are determined by theωempirical graphs. ThenGGandGVcan be calculated as follows.
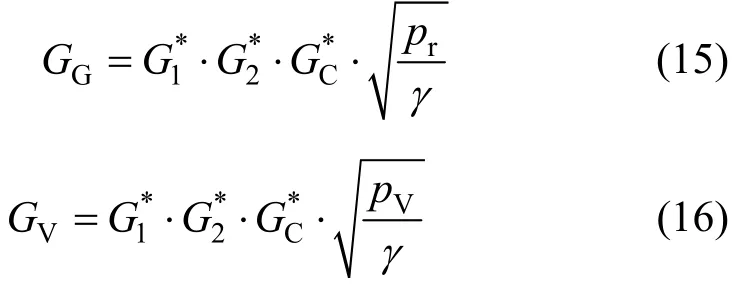
wherer/Vmγ=. The calculation results are listed in Tables 3 and 4. Under ideal condition,yV=pV/p=0.231, soG=12498 kg·m-2·s-1.
In the arithmetic calculation method, the critical pressure ratioηCis determined from Eq. (17) by iteration [13]. Whenη>ηC, the relief flow is in sub-critical state,GGorGVis calculated by Eq. (18). If the relief flow is in critical state,GGorGVis calculated by Eq.(19). By trial and error method, the critical pressure ratio for incompressible gas system and vapor system are obtained as 0.4 and 0.88, respectively. Form Eq. (19),the final results areGG=14273 kg·m-2·s-1andGV=2916 kg·m-2·s-1. From Eq. (14),G=12563 kg·m-2·s-1.
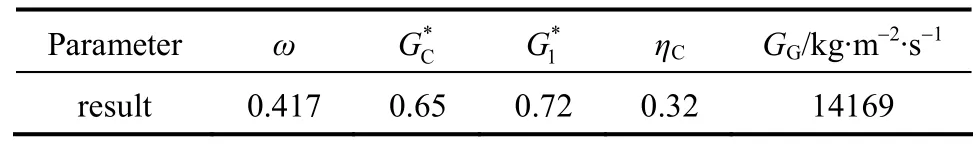
Table 3 Calculation results for GG
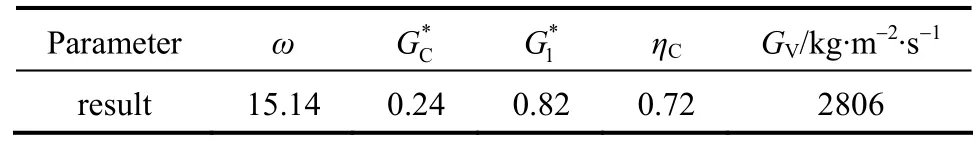
Table 4 Calculation results for GV

As a result the relief sizeAof the storage tank in this case is 0.0159 m2from monograph method and 0.0158 m2from arithmetic method. The relative error is only 0.63%, so the two methods give similar results.
From Eq. (6), it can be inferred that if cooling systems work well, the relief size will be smaller, so it is very important to make cooling systems in good condition in industry.
大学生在成长过程中经常会受到身边不良因素的影响,导致大学生价值观发生偏离,不利于大学生今后的成长〔1〕。因此,在大学思想政治教育中引入“中国梦”宣传教育工作非常重要,其意义主要体现在以下几个方面。
5 CONCLUSIONS
From ARC tests, the DTBP runaway reaction is easy to occur because under adiabatic conditions the initial exothermic temperature of DTBP is 102.6 °C,and the operating temperature in production cell is much higher. The self-accelerating decomposition is rather fast because the time needed to reach the maximum rate is only 273.8 min. The decomposition reaction of DTBP is very drastic, which can be inferred from the maximum self-heating rate (3.095×107°C·min-1)and maximum pressure rising rate (14.762 MPa·min-1).The lgpvs. (1/T) curve during heating and cooling reveals that DTBP runaway involves a hybrid system.
Based on DIERS method, the relief sizes of DTBP runaway system calculated by monograph Omega method and arithmetic Omega method are close, with only 0.63% relative error. From the view of application,the monograph Omega method is more convenient.
NOMENCLATURE
Arelief size, m2
A*pre-exponential factor
c specific heat, kJ·kg-1·K-1
Eaapparent activation energy, kJ·mol-1
G mass flow rate per unit area, kg·m-2·s-1
hfglatent heat, kJ·kg-1
m mass, g
n reaction order
p pressure, MPa
Q vapor or gas generating rate, m3·s-1
q average heat release rate per unit mass of reacting mixture, W·kg-1
R gas constant
T temperature, °C
t time, min
W mass flow rate at releasing, kg·s-1
V volume, m3
y mole friction
α void fraction
γ volume/mass ratio (=V/mr), m3·kg-1
η pressure ratio
ρ density, kg·m-3
φ thermal inertia factor
ω dimensionless parameter criterion
Subscripts
ad adiabatic
b bomb
C critical
e experimental condition
end final exothermic
f sample
G gas
m maximum accumulated pressure
max maximum self-heating rate
r industry condition
s reaction parameter including sample and sample bomb V vapor
0 initial exothermic
1 Jiang, H.L., Qiang, X.M., Fu, Z.M., “Review of emergency relief system for runaway reaction”, Journal of Safety and Environment, 4(2), 86-89 (2004). (in Chinese)
2 Jiang, H.L., Zang, N., Qian, X.M., Fu, Z.M., “Thermal stability of potassium supersulphate and sodium supersulphate”, Journal of Chemical Industry and Engineering (China), 57 (12), 2798-2800(2006). (in Chinese)
3 Townsend, D.I., Tou, J.C., “Thermal hazard evaluation by an accelerating rate calorimeter”, Thermochimica Acta, 37 (1), 1-30 (1980).
4 Zhu, H.Q., Qian, X.M., Fu, Z.M., “Pseudo-inverse metric method-a new method to deal with the adiabatic test data”, Chinese Journal of Explosives & Propellants, 26 (1), 78-80 (2003). (in Chinese)
5 Fu, Z.M., “Evaluating thermal stability for reactive chemical by accelerating rate calorimeter”, Ph. D. Thesis, Beijing Institute of Technology, Beijing (2002). (in Chinese)
6 Huff, J.E., “Emergency venting requirements”, Plant/Operation Progress, 1 (4), 211-229 (1982).
7 Singh, J., “Vent sizing for gas-generating runaway reaction”, Journal of Prevention in the Process Industries, 7 (6), 481-491 (1994).
8 Leung, J.C., “Simplified vent sizing equations for emergency relief requirements in reactors and storage vessels”, AIChE J., 32 (10),1622-1634 (1986).
9 Leung, J.C., Fauske, H.K., “Runaway system characterization and vent sizing based on DIERS technology”, Plant/Operations Progress,6 (2), 77-83 (1987).
10 Fisher, H.G., Emergency Relief System Design Using DIERS Technology—Appendix VI-17(Leung Analytical method II), DIERS/AICHE,USA (1992).
11 Leung, J.C., “The Omega method for discharge rate evaluation”, In:International Symposium on Runaway Reaction and Pressure Relief Design, AICHE, Denver, USA, 367-393(1995).
12 Mcintosh, R.D., Nolan, P.F., “Review of the selection and design of mitigation systems for runaway chemical reactions”, Journal of Loss Prevention in the Process Industries, 14 (2), 27-42 (2001).
13 Gerald, W.B., “Emergency relief system (ERS) design: An integrated approach using DIERS methodology”, Process Safety Process, 14(2), 93-106 (1995).
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Phenol Oxidation by Combined Cavitation Water Jet and Hydrogen Peroxide*
- Effect of Return Sludge Pre-concentration on Biological Phosphorus Removal in a Novel Oxidation Ditch*
- Separation of α-Tocopherol with a Two-Feed Simulated Moving Bed*
- Experimental and CFD Studies on the Performance of Microfiltration Enhanced by a Turbulence Promoter*
- Pervaporation of Aqueous Solution of Acetaldehyde Through ZSM-5 Filled PDMS Composite Membrane*
- Effects of CO2 Dilution on Methane Ignition in Moderate or Intense Low-oxygen Dilution (MILD) Combustion: A Numerical Study*
