Chemical Modeling of Nesquehonite Solubility in Li + Na + K + NH4 +Mg + Cl + H2O System with a Speciation-based Approach*
2012-02-14WANGDaoguang王道广andLIZhibao李志宝
WANG Daoguang (王道广) and LI Zhibao (李志宝)**
Key Laboratory of Green Process and Engineering, National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
1 INTRODUCTION
Nesquehonite (MgCO3·3H2O), one of important mineral compounds of magnesium [1-5], has recently attracted interest due to its good filtration characteristics as a target precipitate in high purity MgO production process [6-8]. Additionally, MgCO3·3H2O is emphasized for its potential role as a CO2-sequestering medium and as a means of disposing Mg-rich wastewater [9-11]. Nesquehonite is usually synthesized through a reactive crystallization (so called precipitation) process by the reaction of MgCl2-rich brine with precipitators, i.e. Na2CO3or (NH4)2CO3, yielding the suspensions with the solutions containing LiCl, NaCl,KCl, NH4Cl, and MgCl2[6-9, 10]. In the crystallization process, the properties of the target solid, namely composition, crystalline, particle size, morphology,cleanliness and stability, are determined by the solid-liquid equilibria (SLE) in the precipitating system [12]. The solubility and stability of nesquehonite in Li + Na + K + NH4+ Mg + Cl + H2O system have been experimentally investigated in our lab [13, 14]. At this point, an accurate comprehensive thermodynamic model capable of predicting nesquehonite solubility in Li + Na + K + NH4+ Mg + Cl + H2O system is desirable and invaluable to obtain crystal nesquehonite with specific/desired properties. Furthermore, the reliable solubility estimation of nesquehonite in the highly concentrated chloride solutions is also an important requirement in developing, designing and optimizing the industrial processes involved in nesquehonite precipitation.
Some work has been done to model the solubility of minerals with some attention given to solubility of nesquehonite. Harvie et al. [15] developed a thermodynamic model for the Na + K + Mg + Ca + H + Cl +SO4+ OH + HCO3+ CO3+ CO2+ H2O system at 298.15 K. Their model successfully describes the solubility of nesquehonite in MgSO4solution at 298.15 K while the inability is observed to represent the solubility of nesquehonite in MgCl2solution.Königsberger et al. [2] established a low-temperature model for the Na2CO3+ MgCO3+ CaCO3+ H2O system below 298.15 K to estimate nesquehonite solubility in the presence of CO2at 101.325 kPa. Marion [16]further extended the low-temperature model to the Na + K + Mg + Ca + H + Cl + SO4+ OH + HCO3+ CO3+CO2+ H2O system. However, limited work has been carried out for a concentrated Li + Na + K + NH4+Mg + Cl + H2O system (I > 10 mol·kg-1) involved in the nesquehonite crystallization process. Therefore,more modeling work is required in order to predict the solubility of nesquehonite in a highly concentrated Li + Na + K + NH4+ Mg + Cl + H2O brine system.
Modeling the solubility of nesquehonite in chloride solutions is a challenging task mainly because the strong non-ideal solution behavior and the formation of ion complex, i.e.[17-22]. It seems that a hybrid modeling approach of ion-association (i.e., speciation using various complexes) and ion-interaction models may be the best to model such complex aqueous systems. Indeed, the speciation-based chemical modeling approach has been successfully used to represent solubility of sparingly soluble solids in various concentrations of chloride-sulphate-carbonate solutions [23-27].Thus, the speciation approach may be adopted to correlate and predict the solubility of nesquehonite in this work.
The objective of this work is to establish an accurate model for predicting nesquehonite solubility and describing the speciation chemistry in the Li+Na+K-NH4-Mg-Cl-H2O system up to 10 mol·kg-1at ambient temperature. The speciation modeling approach combined with Pitzer activity coefficient model embedded in Aspen PlusTMplatform is adopted. The equilibrium constants of nesquehonite and Mg-bearing species are evaluated by Van’t Hoff equation. Pitzer equation parameters of MgHCO3-Cl, MgHCO3-NH4,Na-HCO3-Cl, and Na-CO3-Cl interactions are obtained by correlating solubility data. These new parameters are incorporated in Aspen platform to construct a new chemical model for representing the nesquehonite solubility in the concentrated Li + Na + K + NH4+Mg + Cl + H2O system at temperatures ranging from 288.15 to 308.15 K. Finally, the new model can aid in explaining the solubility behavior of nesquehonite in Li + Na + K + NH4+ Mg + Cl + H2O system.
2 MODELING METHODOLOGY
2.1 Chemical equilibria
In nesquehonite-saturated aqueous electrolyte systems, two types of reaction occur: partial dissolution of nesquehonite and association (speciation) of ionic species. Thus, the solubility of nesquehonite (s) is equal to the sum of the molatilies of the free magnesium ion Mg2+and the associated Mg-bearing species, i.e.

Consequently, the solubility of nesquehonite is governed by the following four equilibrium reactions
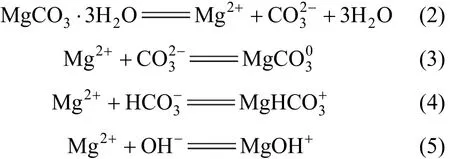
The thermodynamic equilibrium constants for reactions (2) to (5) are

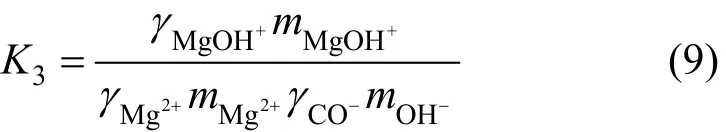
where Kspis the solubility product constant of nesquehonite, K1, K2and K3the association constants ofand MgOH+, respectively, m and γ are the molality and activity coefficient for corresponding species, and awis the activity of water.
By solving Eqs. (1), (6)-(9), we obtain the solubility of nesquehonite

In the MgCl2-containing media, however, the total (Mg) does not represent the nesquehonite solubility,which becomes s=total (C), i.e., s is taken as the sum of all C-bearing species.

Therefore, the following dissociation equilibria should be considered to calculate the nesquehonite solubility in these systems.

The thermodynamic equilibrium constants for reactions (12) and (13) are
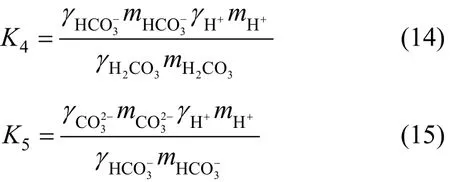
where K4and K5are the dissolution constants of H2CO3and, respectively.
By solving Eqs. (6)-(8), (11), (14) and (15), we obtain the solubility of nesquehonite
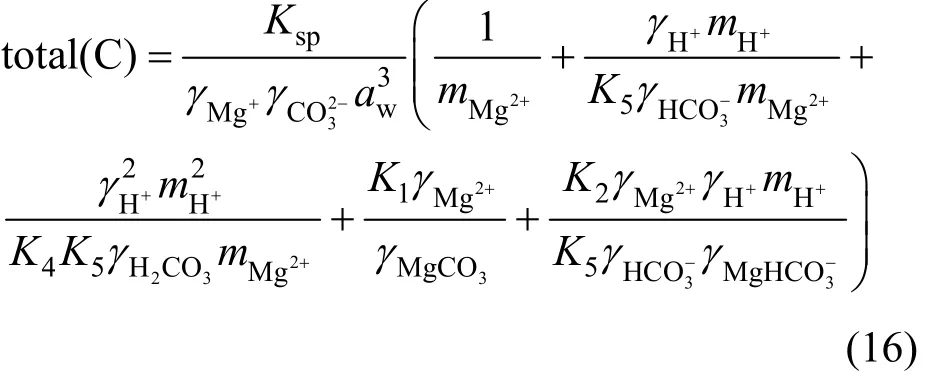
From Eqs. (10) and (16), it is apparent that the calculation of nesquehonite solubility requires the equilibrium constants for all reactions including nesquehonite dissolution and ion complex association in the system of interest, the activity coefficients aqueous species, as well as the activity of water. Therefore, the success in modeling is heavily dependent on the calculation accuracy of the thermodynamic parameters above.
2.2 Equilibrium constant
Two methods,i.e. solubility data regression and thermodynamic data calculation, are feasible to access the chemical equilibrium constants. However, the solubility data are not always available. Therefore, the latter is often employed. The solubility product constant for nesquehonite and equilibrium constants for various complex species of interest in this work are calculated with thermodynamic data by following Van’t Hoff equation [28]
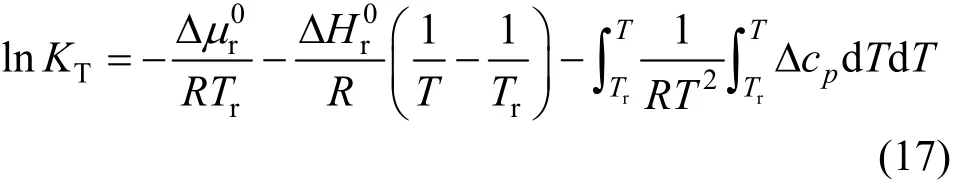
2.3 The Pitzer activity coefficient model
There are different types of activity coefficient models, such as the Pitzer model, the electrolyte NRTL equation, and the Bromely-Zemaitis model.Among these models, the Pitzer ion-interaction model has been successfully used for highly concentrated electrolytes (up to 30 mol·kg-1) and elevated temperature (up to 473 K) to predict the osmotic coefficient and the activity coefficients of chemical species[29], and is subsequently employed in this work. The model has already been discussed in several publications [30, 31], and need not be repeated here. This
model gives expressions for the excess Gibbs energy and hence for the logarithms of the activity coefficients and the osmotic coefficient of electrolyte mixtures in terms of six types of empirical parameters,i.e.β(0),β(1),β(2),Cφ,θij, andψijk. Provided that their temperature and pressure dependencies are known, these parameters permit the calculation of solubilities in binary, ternary and more complex mixtures at different temperatures and pressures. For a limited temperature range around the reference temperatureTr=298.15 K,the binary interaction parameters may be set at a constant with respect to our preliminary calculations. Additionally, the activity of water,aw, is related to the osmotic coefficient,φ, according to
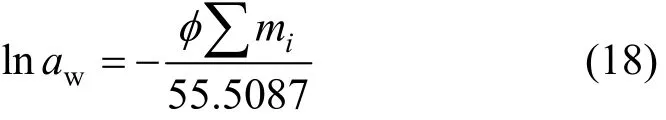
3 RESULTS and DISCUSSION
3.1 Solubility estimation with the existing models
Table 1 Absolute average relative deviation (AARD① AARD(%); NP: number of experimental points. ② Existing model in Aspen Plus./%) between experimental data and calculated results from the model

Table 1 Absolute average relative deviation (AARD① AARD(%); NP: number of experimental points. ② Existing model in Aspen Plus./%) between experimental data and calculated results from the model
Solubility System Temperature/ºC AARD②/% AARD③/% AARD④/%MgCO3·3H2O-H2O 25, 30, 40 41.04 11.07 3.20 MgCO3·3H2O-NaCl-H2O 15, 25, 35 13.23 12.16 2.24 MgCO3·3H2O-KCl-H2O 25 15.63 10.29 1.72 MgCO3·3H2O-LiCl-H2O 25 18.83 10.82 1.40 MgCO3·3H2O-NH4Cl-H2O 15, 25, 35 7.54 11.85 1.94 MgCO3·3H2O-MgCl2-H2O 15, 25 70.76 54.93 26.50 MgCO3·3H2O -NH4Cl-MgCl2-H2O 25, 35 21.54 24.79 2.72
③ Marion model [16]. ④ Model (this work).
The solubility of nesquehonite in water and aqueous solutions of electrolyte,i.e. NaCl, NH4Cl,LiCl, KCl and MgCl2, are estimated first with an Aspen Plus model (software package 11.1) and Marion model [16] by comparing model predictions with experimental data. The absolute average relative deviations (AARD) of the predictions by the two models in all involving systems are listed in Table 1. In general,both models give poor estimates with AARDs more than 10% for almost all of the systems. Even, the AARDs of the predictions by two models for MgCO3⋅3H2O + MgCl2+ H2O system are 70.76% and 54.93%. As an example, Fig. 1 presents a comparison of the model predictions with the experimental measurements [13] for the solubility of nesquehonite in water. The Marion model, with the AARD of 11.07%,underestimates the solubility at temperatures below 303.15 K, but it definitely overestimates the solubility.It can be also seen that the existing model in Aspen grossly underestimates the solubility with the AARD of 41.04%.

Figure 1 Solubility of MgCO3·3H2O in pure water▲ Dong et al. [13]; Model (Aspen); Model (Marion);Model (this work)
The discrepancy between predictions of the existing model and experimental solubilities in the Li +Na + K + NH4+ Mg + Cl + H2O system, as explained later, is mainly attributed to the whole/partial ignorance of Mg-bearing species,i.e.and MgOH+, and their relevant thermodynamic parameters. Given the attractiveness of regression ability incorporated in Aspen PlusTMplatform and its user friendly interface, further work is undertaken with the purpose of improving the solid liquid equilibrium(SLE) estimation capability of Aspenviadetermination and validation of new equilibrium constants and Pitzer model parameters.
3.2 Model parameterization
3.2.1Calculation of equilibrium constants
The dissolution constants of H2CO3andK4andK5, have been thoroughly investigated and proper thermodynamic data have been developed and included in the default databank of Aspen Plus. The present work focuses on the solubility product constant,Ksp, of nesquehonite and the association con-MgOH+, which are inaccurate or absent in the Aspen Plus databank. In order to obtain these constants,μ0,ΔfH0andcpof equilibrium species involved are required by use of the Van’t Hoff equation [Eq. (17)].The relevant thermodynamic data are collected (see Table A1). The thermodynamic data of water is from Zemaitiset al. [32] withcpre-regressed in the following form

withTin Kelvin. Heat capacity of nesquehonite is fitted from data given by Robie [33] with the above equation. Other thermodynamic data listed in Table 2 are from Refs. [34-36] with the heat capacity in the form of
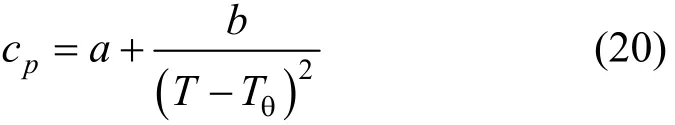
whereTθ=228 K.
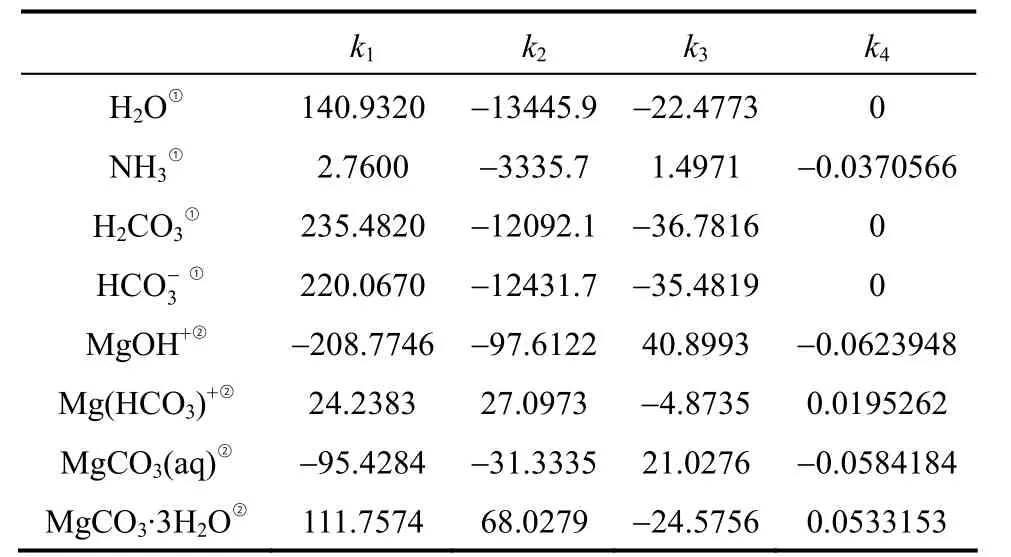
Table 2 Coefficients for aqueous equilibrium constants and nesquehonite solubility product
① Aspen plus. ② This work.
Having compiled the thermodynamic data for all the equilibrium species, the equilibrium constants (Ksp,K1,K2andK3) are determined using Eq. (17). In subsequent calculations, it is conventional to have simple mathematical expressions for the equilibrium constants. Accordingly, these computational results are subjected to regression analysis using an equation of the form

The regressed coefficients are given in Table 2 and highlighted in italic.
3.2.2Pitzer model parameters
Previous investigations for modeling mineral solubilities in concentrated Na + K + Mg + Ca + Cl +SO4+ OH + H2O or Na + K + Mg + Ca + H + Cl +SO4+ OH + HCO3+ CO3+ CO2+ H2O systems have been successfully carried out by Pabalan and Pitzer [29],Harvieet al. [15, 23], as well as other researchers [20].Therefore, binary and ternary Pitzer parameters used in the work (as listed in Tables 3 and 4) are largely taken from their work because these parameters are suitable for high ionic strength brines (up to 30 mol·kg-1). All of the binary and ternary parameters for Na+, K+, Mg2+interactions with Cl-are taken from Pabalan and Pitzer [29], and interactions withandfrom Harvieet al. [15] and He and Morse[20]. Binary interaction parameters of LiCl from Song and Yao [37] are adopted for its application in wide LiCl concentrations up to saturation. Binary interaction parameters of NH4Cl and ternaryθandψparameters of4NH+, Mg2+and Cl-from Balarewet al. [38] and Christov [39] are used. It should be mentioned that the Pitzer parameters and solubility data subjected to the following model parameterization are of 298.15 K.
(1) MgCO3⋅3H2O + H2O The solubility data can be well represented only through the use of the new equilibrium constants listed in Table 2 and the binary Pitzer parameters of Mg-HCO3listed in Table 3 for MgCO3⋅3H2O + H2O system. As can be seen from Fig.1, the model predictions are in good agreement with the experimental values with the AARD of 3.2%.
(2) MgCO3⋅3H2O + NH4Cl + H2O It is noted that the hydrolysis reaction of4NH+ions should be included in the model to predict the solubility of nesquehonite in the system.

Table 3 Pitzer binary interaction parameters, β(0), β(1), and Cφ, for Li + Na + K + NH4 + Mg + Cl + CO3 + HCO3 + H2O system at 298.15 K
① This work.

The above hydrolysis reaction is treated as two dissociation reactions, H2O and NH3, for which the equilibrium constants are available in Aspen Plus listed in Table 2. Fig. 2 shows the comparison of model predictions with experimental data for the solubility of nesquehonite in NH4Cl solutions. Better predictions are obtained with including Eq. (22) than excluding it. However, the pronounced deviation is still observed for the former at higher NH4Cl concen-0.3885 and 0.2735, respectively, lead to a significant improvement in the model fitting to experimental data(see Fig. 2) with AARD of 1.94%.
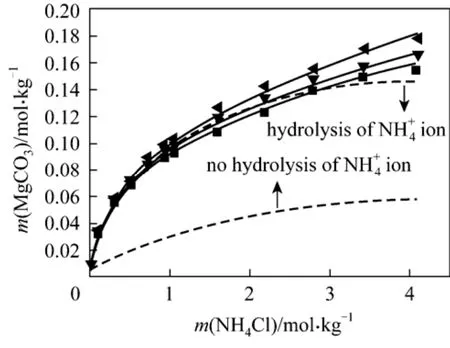
Figure 2 Solubility of MgCO3·3H2O in NH4Cl solutions at different temperatures■ 288.15 K; ▼ 298.15 K; 308.15 K; Model (Aspen);Model (this work)
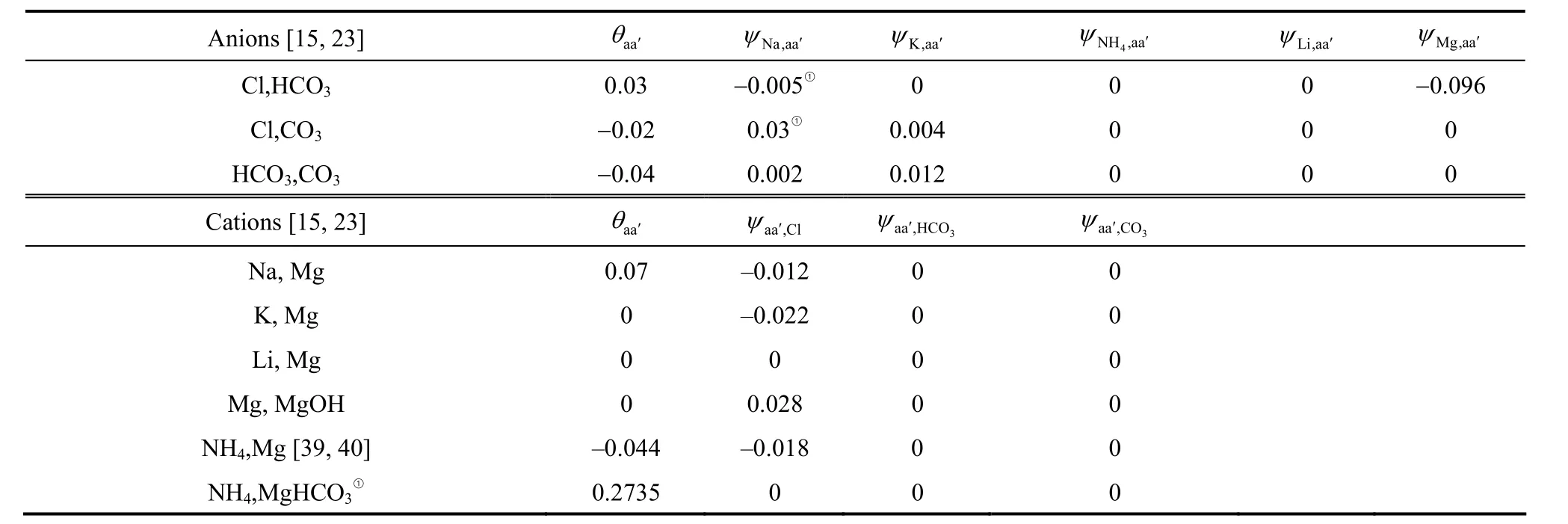
Table 4 The ternary Pitzer interaction parameters, θ and ψ, for Li + Na + K + NH4 + Mg + Cl + CO3 + HCO3 + H2O system at 298.15 K
① This work.

Figure 3 Solubility of MgCO3·3H2O in NaCl solutions at different temperatures■ 288.15 K; ▲ 298.15 K; 308.15 K; 298.15 K (Aspen);Model (this work)
(3) MgCO3⋅3H2O + NaCl + H2O In the system,having values of 0.03 and -0.005, respectively, are obtained by regressing the nesquehonite solubility at 298.15 K. The regressed result is plotted in Fig. 3 with AARD of 2.24%.
(4) MgCO3⋅3H2O + KCl + H2O and MgCO3⋅3H2O +LiCl + H2O The solubility of nesquehonite can be well represented by use of parameters presented above for the two systems. As can be seen from Fig. 4, the model predictions are in good agreement with the experimental values with the AARD of 1.72% and 1.40%.
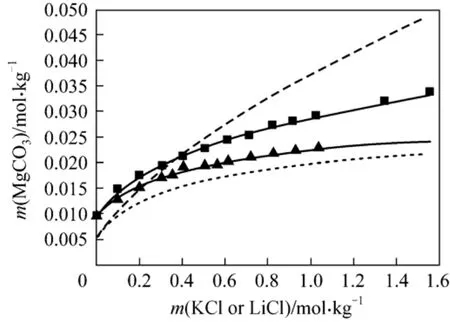
Figure 4 Solubility of MgCO3·3H2O in KCl and LiCl solutions at 298.15 K■ LiCl; ▲ KCl; LiCl (Aspen); KCl (Aspen);Model (this work)
(5) MgCO3⋅3H2O + MgCl2+ H2O As aforementioned, large deviations of 70.76% and 54.93%between predications and experimental data in such system are observed by use of the existing models. It might indicate the difficulty in modeling the nesquehonite solubility in such common ion system. Preliminary examination shows that the solubility of nesquehonite could not be fitted very well with orThe best fitting with AARD of 26.50% is obtained using the parameters listed in Tables 2 and 3 (see Fig.5), indicating significant improvement in comparison with the existing models as shown in Table 1.
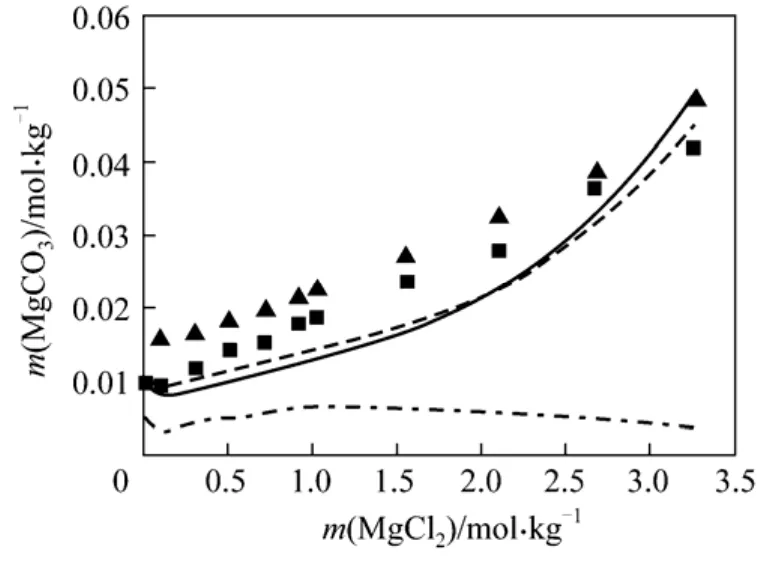
Figure 5 Solubility of MgCO3·3H2O in MgCl2 solutions at 288.15 and 298.15 K■ 288.15 K; ▼ 298.15 K; 298.15 K (Aspen); 298.15 K(this work); 298. 15 (this work)
The predictions with the existing model of Aspen at 298.15 K are also plotted for comparison in Figs. 2-5.It is noticeable that the prediction of the newly developed model has been greatly improved by comparing with the existing models.
3.3 Validation of the model
After successfully modeling the solubility data of nesquehonite in the concentrated NH4+ Na + K + Li +Mg + Cl system at 298.15 K, the resulting model is further validated by comparing model predictions with experimental data not used in model parameterization.To accomplish the task, the solubility of nesquehonite in three binary systems, NaCl + H2O, NH4Cl + H2O and MgCl2+ H2O, at 288.15 and 308.15 K and in the ternary NH4Cl + MgCl2+ H2O system are predicted.In Figs. 2, 3 and 5, 6, the model with the new parameters is evaluated against experimental solubility data.Excellent agreement between new model predictions and experimental data is observed for all the four cases (except for MgCl2). It is noteworthy to point out that neither temperature-dependent parameters nor MgCl2were involved when the new model parameters were determined.
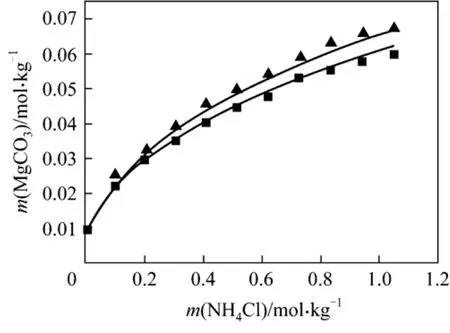
Figure 6 Solubility of MgCO3·3H2O in mixed solutions(NH4Cl + MgCl2) at 298.15 and 308.15 K■ 298.15 K; ▲ 308.15 K; Model (this work)
3.4 Model application: solubility behavior of nesquehonite in chloride media
In order to better appreciate the effect of various chloride salts,i.e. NaCl, NH4Cl, KCl, LiCl, and MgCl2, on the solubility behavior of nesquehonite in aqueous solutions, the predicted solubility data with the new model in various chloride solutions at 298.15 K are summarized in Fig. 7. It is noted that the chloride salts affect the solubility of nesquehonite in a complex way. The effect is stronger as the charge-mass ratio increases for alkali metals,i.e. as we move from KCl to NaCl to LiCl. The solubility of nesquehonite is mildly affected by MgCl2in concentration below 2 mol·kg-1, and then it increases in higher concentration range. Of all salts investigated, NH4Cl seems to enhance the solubility of nesquehonite most.

Figure 7 Effect of various chloride salts on the solubility of nesquehonite at 298.15 K on the basis of new model estimates 1—NaCl; 2—KCl; 3—LiCl; 4—MgCl2; 5—NH4Cl
As aforementioned, it can be derived from Eqs.(10) and (16) that the solubility of nesquehonite is determined by the activity coefficients, Mg/CO3-bearing speciation and H+/OH-, as well as the equilibrium constants. Furthermore, at a given temperature, the equilibrium constants are invariable. Thus, we decided to investigate the Mg/CO3-bearing speciation distribution, activity coefficients, and pH in various chloride solutions with the aid of the newly developed model to further explain the effect of various chloride salts on nesquehonite solubility.
3.4.1The behavior of Mg/CO3-bearing speciation in chloride solutions
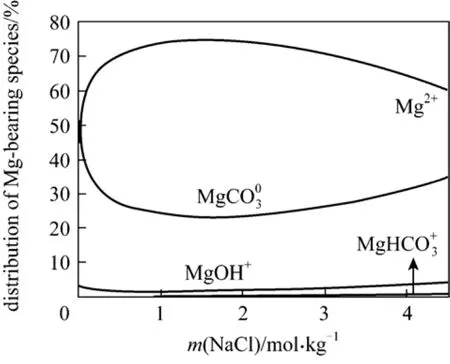
Figure 8 Magnesium speciation at 298.15 K in nesquehonite-saturated NaCl brine

Figure 9 Magnesium speciation at 298.15 K in nesquehonite-saturated NH4Cl brine
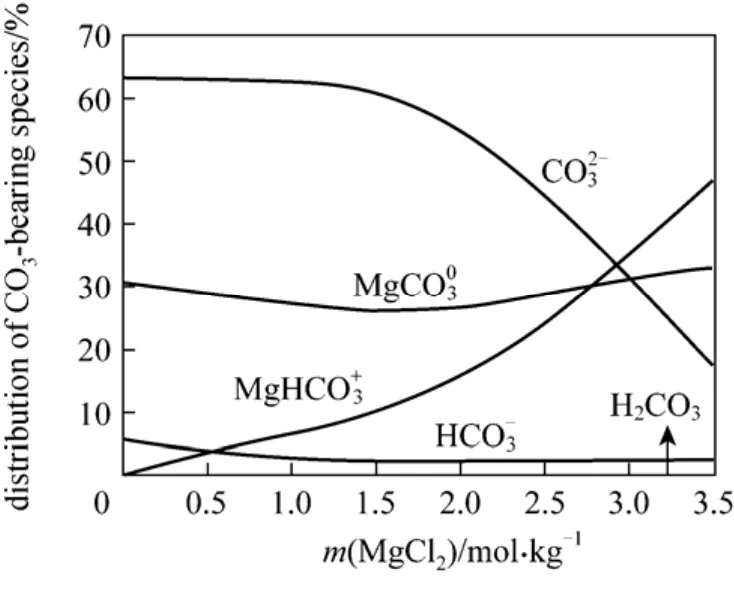
Figure 10 Carbon speciation at 298.15 K in nesquehonite-saturated MgCl2 brine
The distribution of Mg/CO3-bearing species in NaCl, NH4Cl, or MgCl2solutions saturated with nesquehonite is shown in Figs. 8-10, while the distribution of Mg/CO3-bearing species for LiCl and KCl solutions is not shown since they are very similar to Fig. 8. In the case of NaCl (Fig. 8), it is obvious that the magnesium ion (Mg2+) dominates the nesquehonite solubility while the magnesium carbonate neutral species,, is a moderately important species. Theion seems to be negligible. A dramatic variation is observed in the relative abundance of the four Mg-bearing species with addition of NH4Cl(Fig. 9) up to 0.3 mol·kg-1. Theion, instead, becomes moderately important at higher NH4Cl concentrations. The Mg2+ion still controls the nesquehonite solubility in the whole concentration range of NH4Cl. MgOH+becomes negligible in the case.
The distribution of carbonate-bearing species is given in Fig. 10 for the case of MgCl2.is modestly important C-bearing species in the whole MgCl2concentration range. Following a plateau, the concentration of dominantspecies dramatically decreases in the concentration range of MgCl2above~1.5 mol·kg-1. It is noticeable that the abundance of MgHboosts with the increment of MgCl2, and becomes predominant at higher MgCl2concentrations.This is due to a twofold effect of magnesium ion on the dissolution of nesquehonite [reversing Eq. (2)] and the formation of Mg-bearing species [facilitating Eqs.(3)-(5)].
3.4.2The behavior of activity coefficients in chloride solutions
Solubility of nesquehonite is fundamentally determined by the dissolution reaction, Eq. (2). According to the thermodynamic definition [Eq. (6)], the solubility product constant can be given by
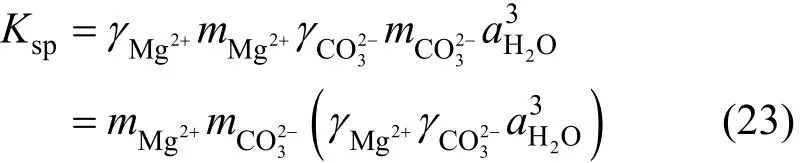
In order to reveal the effect of ion interaction on nesquehonite solubility, the activity coefficient product(as calculated with the aid of the newly developed model) as a function of chloride salt concentration at 298.15 K is plotted in Fig. 11. Thevalue reaches a minimum (at ~2-3 m),and then gradually increases with NaCl concentration.This minimum corresponds to the maximum nesquehonite solubility (see Fig. 2). However, thesalt solutions except for NaCl case. The behavior ofvalues mirrors the sharp of solubility curve for the case of NaCl, LiCl, KCl and NH4Cl (see
value and nesquehonite solubility in MgCl2case is due to the complexity caused by the dissociation and association involving Mg2+ion.
3.4.3The behavior of pH values in chloride solutionsFigure 12 demonstrates the pH values (as calculated with the aid of the newly developed model) of nesquehonite-saturated solutions containing chloride salts. The pH values vary mildly from 10.50 to 10.82 for the system containing alkali metal salts. The pH values are preferred for the existence ofcases. Thus, the dominantion associated with the dominant Mg2+ion in the Mg-bearing species results in the modestly importantion,dominant in C-bearing species in the alkali metals species in these solutions. The pH value in MgCl2case is modest due to the formation of MgOH+[as shown in Eq. (4)],the abundance of which is similar toin aqueous solution. The variation of pH is most significant in the case of NH4Cl. It can be seen that the pH value sharply decreases to 9 with addition of NH4Cl to 0.075 mol·kg-1, and then gradually to 8 with further increase of NH4Cl concentration to 4.5 mol·kg-1. The dramatic decrease of pH is due to the hydrolysis ofion [as shown in Eq. (22)]. In the NH4Cl case,the pH value ranging from 8 to 9 is preferred for the formation ofbecomes dominant in C-bearing species andMg is subsequently the modestly important species in-containing solutions.

Figure 11 Calculated values of 3 as a function of concentration for various chloride salts at 298.15 K 1—NaCl; 2—KCl; 3—LiCl; 4—MgCl2; 5—NH4Cl

Figure 12 Calculated pH as a function of concentration for various chloride salts at 298.15 K
4 CONCLUSIONS
A chemical model to describe the chemistry and solubility of nesquehonite in concentrated chloridecontaining brine has been successfully developed on the basis of Pitzer model embedded in Aspen Plus platform. The model incorporates equilibrium constants and new Pitzer parameters obtained in this work, as well as those model parameters available in literature.The accurate equilibrium constants of solid and aqueous species,i.e. nesquehonite,and MgOH+, are judicially obtained with the published thermodynamic data. The prediction ability of the new model is greatly improved, superior to those presented in other studies. The modeling results indicate that the new thermodynamic model on the basis of Pitzer model embedded in Aspen Plus platform, after improvements made in this work, is a good tool to describe nesquehonite solubility behavior and calculate solution speciation in concentrated NH4+ Na + K + Li + Mg + Cl + H2O system at ambient temperature. With the help of the model,the difference of solubility behavior for nesquehonite in various chloride solutions is explained, based on the formation of,etc., pH and activity coefficient changes.
1 Hänchen, M., Prigiobbe, V., Baciocchil, R., Mazzotti, M., “Precipitation in the Mg-carbonate system-effects of temperature and CO2pressure”,Chem.Eng.Sci., 63, 1012-1028 (2008).
2 Königsberger, E., Königsberger, L.C., Gamsjäger, H., “Low-temperature thermodynamic model for the system Na2CO3-MgCO3-CaCO3-H2O”,Geochim.Cosmochim.Acta, 63, 3105-3119 (1999).
3 Hopkinson, L., Rutt, K., Cressey, G., “The transformation of nesquehonite to hydromagnesite in the system CaO-MgO-H2O-CO2:An experimental spectroscopic study”,The Journal of Geology, 116,387-400 (2008).
4 Kloprogge, J.T., Martens, W.N., Nothdurft, L., Duong, L.V., Webb,G.E., “Low temperature synthesis and characterization of nesquehonite”,Journal of Materials Science Letters, 22, 825v829 (2003).
5 Giester, G., Lengauer, C.L., Rieck, B., “The crystal structure of nesquehonite, MgCO3·3H2O, from Lavrion, Greece”,Mineralogy and Petrology, 70, 153-163 (2000).
6 Wang, Y., Li, Z.B., Demopoulos, G.P., “Controlled precipitation of nesquehonite by the reaction of MgCl2with (NH4)2CO3at 303 K”,J.Cryst.Growth, 310, 1220-1227 (2007).
7 Cheng, W.T., Li, Z.B., “Precipitation of nesquehonite from homogeneous supersaturated solutions”,Cryst.Res.Technol., 44, 937-947(2009).
8 Cheng, W.T., Li, Z.B., Demopoulos, G.P., “Effect of temperature on the preparation of magnesium carbonate hydrates by reaction of MgCl2with Na2CO3”,Chin.J.Chem.Eng., 17, 661-669 (2009).
9 Park, A.A., “Carbon dioxide sequestration: Chemical and physical activation of aqueous carbonation of Mg-bearing minerals and pH swing process”, Ph.D. Dissertation, The Ohio State University, USA(2005).
10 Ferrini, V., Vito, C.D., Mignardi, S., “Synthesis of nesquehonite by reaction of gaseous CO2with Mg chloride solution: Its potential role in the sequestration of carbon dioxide”,J.HazardousMaterials, 168,832-837 (2009).
11 Ballirano, P., Vito, C.D., Ferrini, V., Mignardi, S., “The thermal behavior and structural stability of nesquehonite, MgCO3·3H2O, evaluated byin situlaboratory parallel-beam X-ray powder diffraction:New constraints on CO2sequestration within minerals”,J.HazardousMaterials, 178, 522-528 (2010).
12 Demopoulos, G.P., “Aqueous precipitation and crystallization for the production of particulate solids with desired properties”,Hydrometallurgy, 96, 199-214 (2009).
13 Dong, M., Li, Z.B., Mi, J.G., Demopoulos, G.P., “Solubility and stability of nesquehonite (MgCO3·3H2O) in mixed NaCl, KCl, MgCl2and NH4Cl solutions”,J.Chem.Eng.Data, 53, 2586-2593 (2008).
14 Dong, M., Li, Z.B., Mi, J.G., Demopoulos, G.P., “Solubility and stability of nesquehonite (MgCO3·3H2O) in mixed NaCl + MgCl2,NH4Cl + MgCl2, LiCl and LiCl + MgCl2solutions”,J.Chem.Eng.Data, 54, 3002-3007 (2009).
15 Harvie, C.E., Møller, N., Weare, J.H., “The prediction of mineral solubilities in natural waters: Na-K-Mg-Ca-H-Cl-SO4-OH-HCO3-CO3-CO2-H2O system to high ionic strengths at 25 °C”,Geochim.Cosmochim.Acta, 48, 723-751 (1984).
16 Marion, G.M., “Carbonate mineral solubility at low temperatures in the Na-K-Mg-Ca-H-Cl-SO4-OH-HCO3-CO3-CO2-H2O system”,Geochim.Cosmochim.Acta, 65, 1883-1896 (2001).
17 Nakayama, F.S., “Magnesium complex and ion-pair in MgCO3-CO2solution system”,J.Chem.Eng.Data, 16, 178-181 (1971).
18 McGee, K.A., Hostetler, P.B., “Studies+in the system MgO-SiO2-CO2-H2O. IV: The stability of MgOH from 10 to 90 °C”,Am.J.Sci., 275, 304-317 (1975).
19 Millero, F.J., Thurmond V., “The ionization of carbonic acid in Na-Mg-Cl solutions at 25 °C”,J.Solution Chem., 12, 401-412(1983).
20 He, S., Morse, J.W., “The carbonic acid system and calcite solubility in aqueous Na-K-Ca-Mg-Cl-SO4 solutions form 0 to 90 °C”,Geochim.Cosmochim.Acta., 57, 3533-3555 (1993).
21 Siebert, R.M., Hostetler, P.B. , “The stability of the magnesium carbonate ion pair from 10° to 90 °C”,Am.J.Sci., 277, 716-734(1977).
22 Bauman, J.E.Jr., “Thermodynamic measurements of carbonate equilibria involving metal ions”,Inf.Circ.Bur.MinesU.S.Dep.Inter.No, 8853, 268-274 (1981).
23 Harvie, C.E., Weare, J.H., “The prediction of mineral solubilities in natural waters: The Na-K-Mg-Ca-Cl-SO4-H2O systems from zero to high concentration at 25 °C”,Geochim.Cosmochim.Acta, 44,981-997 (1980).
24 Li, Z.B., Demopoulos, G.P., “Speciation-based chemical equilibrium model of CaSO4solubility in the H + Na + Ca + Mg + Al + Fe(II) +Cl + SO4+ H2O system”,Ind.Eng.Chem.Res., 46, 6385-6392 (2007).
25 Azimi, G., Papangelakis, V.G., Dutrizac, J.E., “Development of an MSE-based chemical model for the solubility of calcium sulphate in mixed chloride-sulphate solutions”,Fluid Phase Equilibria, 266,172-186 (2008).
26 Azimi, G., Papangelakis, V.G., “Thermodynamic modeling and experimental measurement of calcium sulphate solubility in complex aqueous solutions”,Fluid Phase Equilibria, 290, 88-94 (2010).
27 Azimi, G., Papangelakis, V.G., “The solubility of gypsum and anhydrite in simulated laterite pressure acid leach solutions up to 250 °C”,Hydrometallurgy, 102, 1-13 (2010).
28 Wang, D.G., Li, Z.B., “Modeling solid-liquid equilibrium of NH4Cl-MgCl2-H2O system and its application to recovery of NH4Cl in MgO production”,AIChE J., 57, 1595-1606 (2011).
29 Pabalan, R.T., Pitzer, K.S., “Thermodynamics of concentrated electrolyte mixtures and the prediction of mineral solubilities to high temperatures for mixtures in the system Na-K-Mg-Cl-SO4-OH-H2O”,Geochim.Cosmochim.Acta, 51, 2429-2443 (1987).
30 Pitzer, K.S., “Thermodynamics of electrolytes. I. Theoretical basis and general equations”,J.Phys.Chem., 77, 268-277 (1973).
31 Pitzer, K.S., “Theory: Ion interaction approach”, In: Activity Coefficients in Electrolyte Solutions, CRC Press, Boca Raton, 75-154(1991).
32 Zemaitis, J.F., Clark, D.M., Rafal, M., Scrivner, N.C., Handbook of Aqueous Electrolyte Thermodynamics, Des. Inst. for Phys. Prop.Res., AIChE, New York (1986).
33 Robie, R.A., “The heat capacities at low temperatures and entropies at 298.15 K of nesquehonite, MgCO3·3H2O, and hydromagnesite”,American Mineralogist, 57, 1768-1781 (1972).
34 Shock, E.L., Helgeson, H.C., “Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: Correlation algorithms for ionic species and equation of state predictions to 5 Kb and 1000 °C”,Geochim.Cosmochim.Acta, 52, 2009-2036 (1988).
35 Shock, E.L., Sassani, D.C., Willis, M., Sverjensky, D.A., “Inorganic species in geologic fluids: Correlations among standard molar thermodynamic properties of aqueous ions and hydroxide complexes”,Geochim.Cosmochim.Acta, 61, 907-950 (1997).
36 Johnson, J.W., Oelkers, R.H., Helgeson, H.C., “Supcrt92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous, and reactions from 1 to 5000 bar and 0 to 1000 °C”,Computers and Geosciences, 18, 899-947 (1992).
37 Song, P.S., Yao, Y., “On Pitzer parameters of LiCl and Li2SO4”,Journal of Salt Lake Science, 4, 55-63 (1996).
38 Balarew, C., Christov, C., Valyashko, V., Petrenko, S., “Thermodynamics of formation of carnallite type double salts”,J.Solution Chem., 22, 173-181 (1993).
39 Christov, C., “Thermodynamics of formation of double salts and mixed crystals from aqueous solutions”,J.Chem.Thermodynamics,37, 1036-1060 (2005).
APPENDIX
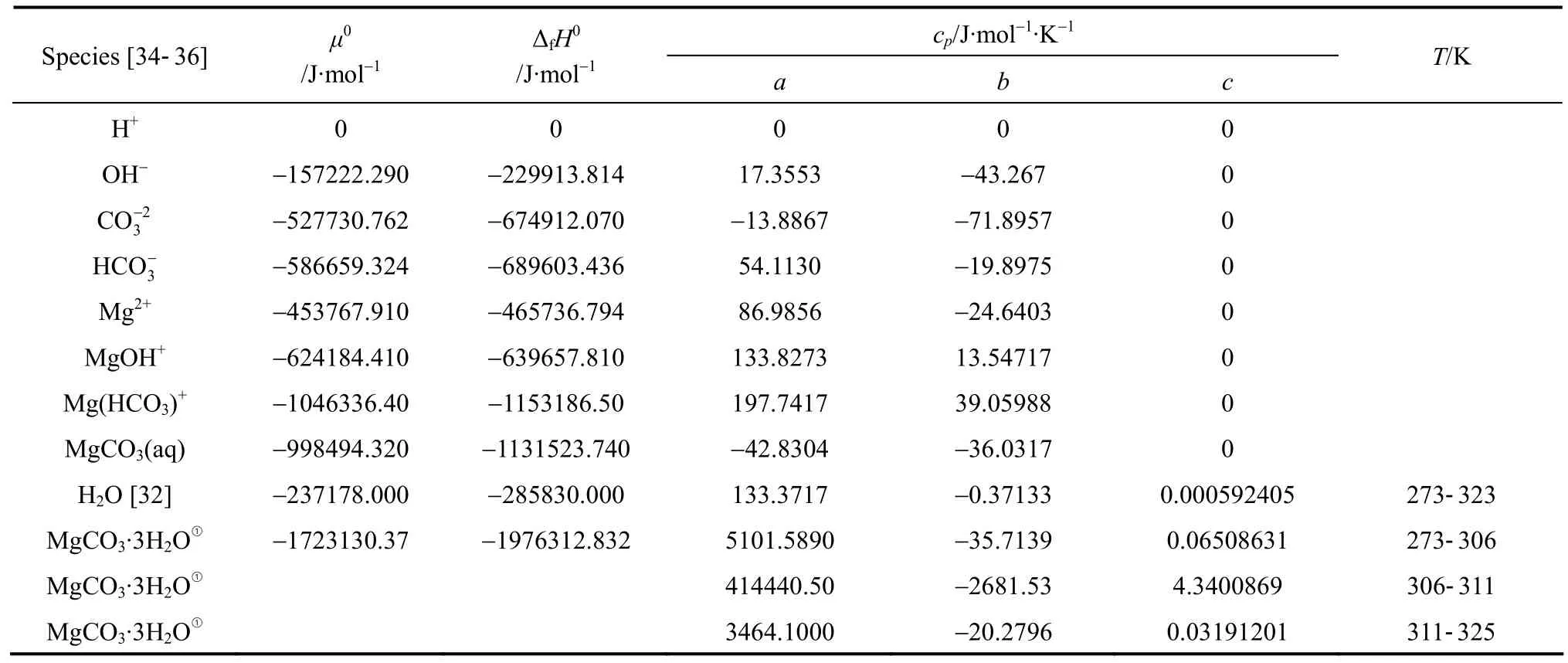
Table A1 Standard-state chemical potentials, enthalpies of formation, and heat capacity of species
① Fitted from data given by Robie [33].
