Doping 25-Atom and 38-Atom Gold Nanoclusters with Palladium
2011-12-12QIANHuifengBARRYEllenZHUYanJINRongchao
QIAN Huifeng BARRY Ellen ZHU Yan JIN Rongchao,*
(1Department of Chemistry,Carnegie Mellon University,Pittsburgh,Pennsylvania 15213,USA; 2Department of Chemistry,University of Pittsburgh,Pittsburgh,Pennsylvania 15260,USA)
1 Introduction
Quantum-sized gold nanoclusters have been intensely pursued in recent years due to their exciting material properties caused by quantum confinement of electrons in the particle when the particle size is in the ultrasmall size regime(<2 nm diameter).1-10The electronic structure of these nanoclusters shows significantly quantized discrete energy levels,10which is in striking contrast with the quasi-continuous energy bands in bulk materials.Due to electron energy quantization,nanoclusters display many interesting properties,such as single-electron optical absorption and chiro-optical properties,fluorescence,intrinsic magnetism,etc.11-19In particular,gold nanoclusters have been found to show remarkable catalytic activity in some chemical reactions.20-23All these new properties demonstrate the promise of this new class of material.
A critical aspect towards understanding the new properties of gold nanoclusters involves the development of synthetic procedures that allow for the synthesis of single sized nanoclusters (i.e.,no size dispersity).1,24We have previously developed a kinetically controlled method for the synthesis of atomically precise,thiolate protected Au25(SR)18clusters.25,26The underlying mechanism has been identified to be a size-focusing process.24Among the Aun(SR)mclusters,the 25-atom cluster has gained wide interest as it is the earliest prepared,molecule-like cluster by Whetten and coworkers;2note that the initial incorrect assignment of Au28(SG)16(where,―SG=glutathione)has been corrected as Au25(SG)18by Tsukuda and coworkers.14With respect to the structure,both anionic and neutral Au25(SR)18clusters are composed of a centered icosahedral,13-atom gold core and an exterior shell of twelve gold atoms.10,11The highly symmetric structure(quasi-D2h)of the Au25cluster largely contributes to its exceptional stability.Fields-Zinna et al.27reported doping of the Au25cluster with palladium and found that mono-Pd doped Au25cluster was formed regardless of the initial ratio of Au and Pd precursors.Recently,Negishi et al.28isolated dodecanethiolate-capped Pd1Au24(SC12H25)18by solvent fractionation and high-performance liquid chromatography (HPLC).Theoretical calculations based on density functional theory(DFT)suggest that the Pd atom should replace the central Au atom in the Au25(SR)18structure,forming a Pd@Au12icosahedral core,which is further capped by an exterior shell of Au12,and the entire cluster is protected by eighteen thio-lates.29-31The Pd-doped Pd1Au24cluster exhibits higher stability than the homogold Au25cluster.28-31It is noteworthy that other metal-doped M1Au24clusters(where,M=Ag,Mn,Pt,etc.)have also been theoretically discussed in DFT works.32-34
It is highly desirable to develop facile protocols that permit the synthesis of monodisperse Pd1Au24(SR)18clusters in order to fully understand the optical,electronic,and catalytic properties.In this work,we report two new methods for preparing mono-palladium doped 25-atom clusters(referred to as Pd1Au24(SC2H4Ph)18).Size exclusion chromatography(SEC)is used to isolate the Pd1Au24(SC2H4Ph)18clusters.The as-obtained clusters are truly monodisperse,evidenced by mass spectrometry and other characterization.The optical and structural properties of Pd1Au24(SC2H4Ph)18,as well as its catalytic activity are investigated in this work.Interestingly,similar central doping behavior is also present in the 38-atom gold cluster which possesses a structure of a biicosahedral Au23core capped by 15 surface Au atoms.Our work is in hope to stimulate wide research interest in this new class ofAun(SR)mnanomaterial.35-52
2 Experimental
2.1 Chemicals
Palladium(II)acetate(Pd(Ac)2,99.9%,Aldrich),tetrachloroauric(III)acid(HAuCl4·3H2O,99.99%,Aldrich),2-phenylethanethiol(PhC2H4SH,99%,Aldrich),sodium borohydride (NaBH4,99.99%,Aldrich),tetraoctylammonium bromide (TOAB,>98%,Fluka),toluene(HPLC grade,99.9%,Aldrich), ethanol(HPLC grade,Aldrich),tetrahydrofuran(THF,HPLC grade,Aldrich),acetonitrile(HPLC grade,99%,Aldrich),and dichloromethane(HPLC grade,99.9%,Aldrich)are used as received.Nanopure water(18.2 MΩ·cm)is used in all experiments that involve water.
2.2 Two-phase synthesis of Pd1Au24(SC2H4Ph)18clusters
The method for preparing thiolate protected,mono-palladium doped Au25clusters was an alteration of the low-temperature protocol for monodisperse Au25clusters in high yield.25In a typical experiment,HAuCl4·3H2O(0.157 g,0.4 mmol), Pd(Ac)2(0.010 g,0.044 mmol),and tetraoctylammonium bromide(TOAB,0.281 g)were combined in a 25 mL tri-neck round bottom flask along with 6 mL of nanopure water and 10 mL of toluene.The mixture was vigorously stirred(~1000 r· min-1)for~15 min to enable TOAB-facilitated phase transfer of the gold and palladium precursors from the aqueous phase to the organic phase(toluene).The clear aqueous phase was then removed using a glass pipette.The toluene phase was cooled in an ice-bath for~30 min under constant,slow stirring (~60 r·min-1).Phenylethanethiol(0.202 mL,1.47 mmol)was added into the flask,and the solution was then slowly stirred for~45 min in the ice-bath.After that,the slow magnetic stirring spend was changed to vigorous stirring(~1000 r·min-1) for several seconds,and a freshly made,ice-cold NaBH4solution(0.169 g dissolved in 6 mL cold water,10:1 molar ratio of NaBH4to the total gold and palladium)was immediately added to the flask.The solution in the flask turned black instantly,indicating the formation of clusters.The reaction was allowed to proceed overnight under constant stirring.
2.3 One-phase synthesis of Pd1Au24(SC2H4Ph)18clusters
This is a modification of the one phase synthesis of Au25(SR)18clusters.26,27HAuCl4·3H2O(0.157 g,0.4 mmol),Pd(Ac)2(0.010 g,0.044 mmol),and TOAB(~0.281 g)were combined in a 25 mL tri-neck round bottom flask along with 15 mL THF solvent.The mixture was vigorously stirred for 15 min.Phenylethanethiol(0.202 mL)was added to the flask at room temperature(r.t.)without changing the stirring speed.The solution color gradually changed from orange to colorless within~15 min. After that,0.169 g NaBH4dissolved in 6 mL of cold water was added to the flask all at once.The solution turned black immediately,indicating the formation of nanoclusters.The reaction was stopped after 3 h.
甲基二磺隆和炔草酯防治小麦田禾本科杂草效果研究……………………………… 于金萍,刘亦学,张 惟,李 琦,白鹏华(83)
2.4 Purification and isolation of Pd1Au24(SC2H4Ph)18clusters
The nanoclusters were obtained from the two-phase and one-phase syntheses by the following process.The solution containing the crude product was first transferred to a 100 mL round bottom flask and dried using a rotary evaporator(~15 min).The dried nanoclusters were washed with ethanol and then collected by centrifugation.This process was repeated for 3 times.The Pd1Au24(SC2H4Ph)18and Au25(SC2H4Ph)18were then extracted from the solids with acetonitrile and the solution was dried on a rotary evaporator.The process of extraction with acetonitrile and subsequent drying of the solution was repeated twice.The nanoclusters were isolated by size exclusion chromatography(SEC)using a PLgel column(gel particle size:3 μm,pore diameter:10.0 nm).SEC was performed on a HPAgilent 1100 HPLC system equipped with a diode array detector (DAD).The mobile phase was CH2Cl2(flow rate:0.5 mL· min-1).The retention time of Pd1Au24(SC2H4Ph)18is~15 min (peak time).To collect the clusters,the eluate from 14.2 to 14.9 min was collected.
2.5 Characterization of clusters
UV-Vis spectra of the clusters(dissolved in CH2Cl2)were acquired on a Hewlett-Packard(HP)Agilent 8453 diode array spectrophotometer at room temperature.Matrix-assisted laser desorption ionization(MALDI)mass spectrometry was performed with a PerSeptiveBiosystems Voyager DE super-STR time-of-flight(TOF)mass spectrometer.Trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenyldidene]malononitrile(DCTB) was used as the matrix in MALDI analysis.45Typically,1 mg matrix and 0.1 mg analyte stock solution were mixed in 100 μL CH2Cl2.10 μL solution was applied to the steel plate and then air-dried prior to MALDI analysis.Laser desorption ionization(LDI)mass spectrometry analysis was done on the same mass spectrometer(without the addition of DCTB matrix).
3 Results and discussion
3.1 Synthesis and isolation of Pd1Au24(SR)18clusters
We previously reported the two-phase and one-phase syntheses of Au25(SR)18clusters in high yield.25,26Several variations have also been reported recently.27,42In this work,we have modified these two protocols to prepare mono-palladium doped 25-atom nanoclusters.The main difference between these two methods lies in the solvent(s)used.26In the two-phase protocol, HAuCl4is dissolved in aqueous phase,and phase transferred to toluene phase with the assistance of tetraoctylammonium bromide(TOAB).In the one-phase protocol,HAuCl4is directly dissolved in THF;TOAB is still used,but to facilitate the formation ofAu25clusters.27
Fig.1(A)(profiles a and b)shows the wide-range MALDI-mass spectra of crude Pd-Au clusters synthesized by the one-phase and two-phase procedures,respectively,and Fig.1 (B)shows the zoom-in spectra.The peaks of m/z 7394 and 6054 are from the ionized Au25(SR)18(where,R=C2H4Ph hereafter)and its fragment,45and the peak of m/z 7303 is assigned to Pd1Au24(SR)18(theoretical formula weight:7303.2).28The yield of Pd1Au24clusters is higher in the two-phase procedure(Fig.1 (B),spectrum a)than that of the one-phase method(Fig.1(B), spectrum b).The ubiquitous existence of Pd1Au24clusters suggests the particular stability of this cluster.28It is probably because that the Pd atom in the center position has a larger interaction energy with the Au24(SR)18cage than the central gold atom does in the case ofAu@Au24(SR)18.29
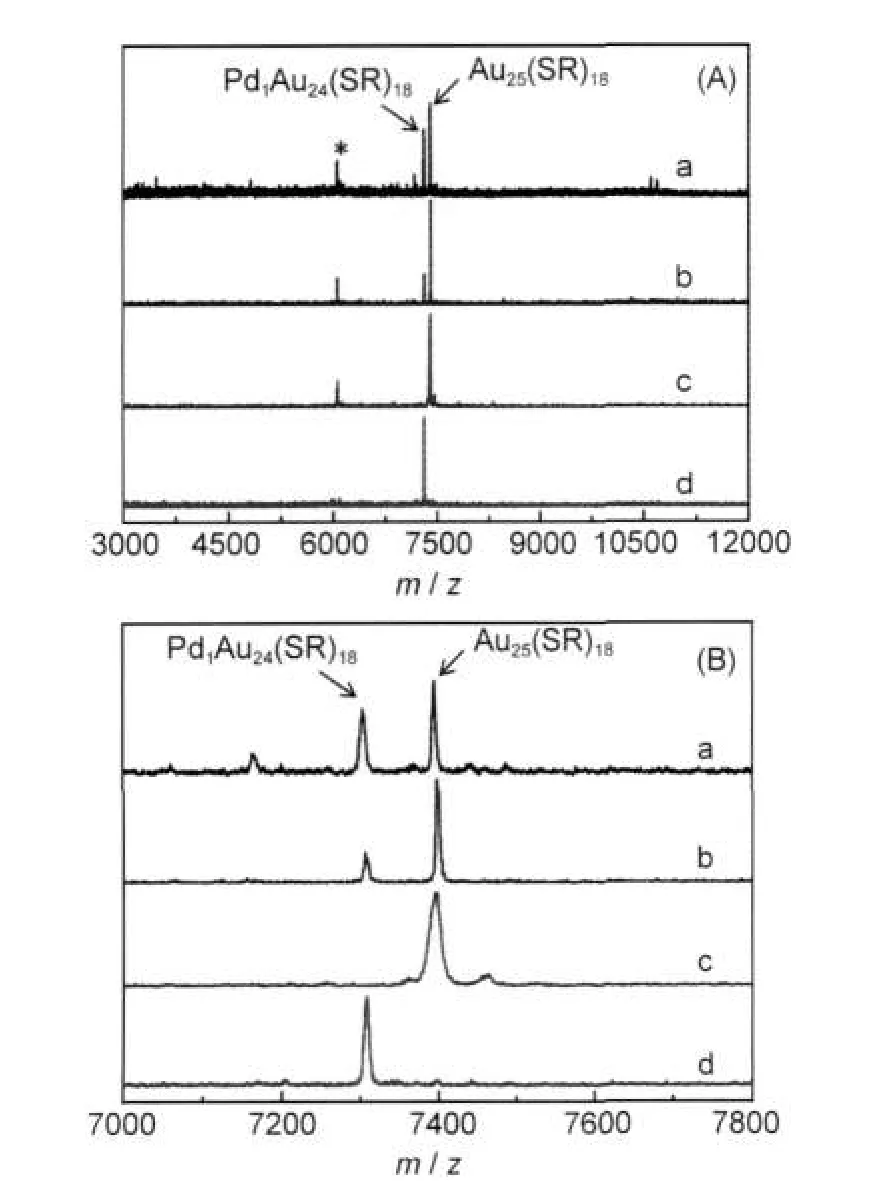
Fig.1 (A)Negative-mode MALDI mass spectra(m/z 3000-12000) of clusters and(B)zoom-in spectra in the range of m/z 7000-7800(a)synthesized by the two-phase procedure,(b)synthesized by the one-phase procedure,(c)SEC isolatedAu25(SR)18cluster,(d)SEC isolated Pd1Au24(SR)18cluster,where R=C2H4Ph.
In our previous work,size exclusion chromatography(SEC) has been used to successfully separate Au38(SR)24and Au40(SR)24clusters.39Herein,SEC is performed to isolate Pd1Au24from the mixture of Pd1Au24and Au25clusters.Fig.2 shows the size exclusion chromatogram of mixed clusters prepared by the two-phase procedure.In the chromatogram,two peaks are observed,which correspond to the two clusters observed in the MALDI mass spectrum.The eluate at the retention time 13.0-13.4 min and 14.2-14.9 min were collected,respectively.The isolated samples were further characterized by MALDI-MS. Profile c(Fig.1(A))is the first fraction and profile d is the second fraction.In profile c,the mass spectrum displays an intense peak at m/z 7394 and a weak peak at m/z 6054;they are assigned to Au25(SR)18(theoretical m/z:7393.7)and its fragment Au21(SR)14(theoretical m/z:6057.2).In profile d,there is only one intense peak at m/z 7303,corresponding to intact Pd1Au24(SC2H4Ph)18(theoretical m/z:7303.4).The observation of a single peak in the wide range from m/z 3000 to 12000 indicates the high purity of the Pd1Au24(SR)18clusters isolated by SEC.The high-resolution MALDI mass spectrum of Pd1Au24(SR)18is shown in Fig.3.The observed isotope pattern of m/z 7303 is superimposable to the simulated pattern of Pd1Au24(SR)18, confirming the assignment of the formula.
3.2 Probing the structure of Pd1Au24(SR)18clusters
On the basis of the Au25(SR)18structure,there are three possible doping sites for the palladium atom:in the center of the icosahedral Au13core(Scheme 1),at the icosahedral surface,and in the exterior Au12shell.DFT calculations28-31show that the center-doped Pd1Au24(SR)18has the highest stability compared to the other two doping sites.Here we provide experimental evidences to support the central doping of Pd in the structure of Pd1Au24(SR)18and its overall core-shell structure similar to that of Au25(SR)18.Our experimental evidences include the similar fragments observed in MALDI and LDI mass spectra of Pd1Au24(SR)18and Au25(SR)18,as well as the information from the optical absorption spectra.
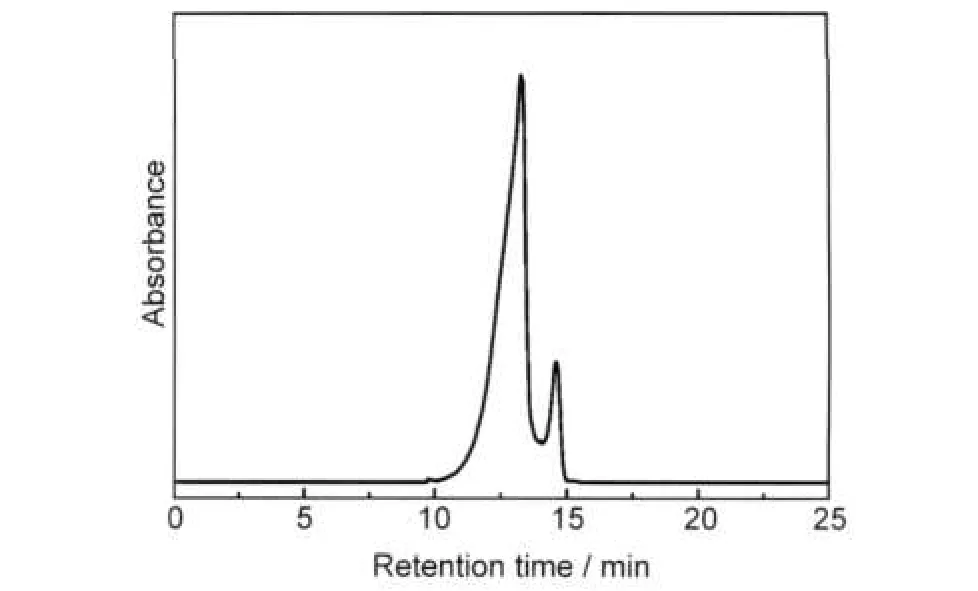
Fig.2 SEC chromatogram of clusters synthesized by the two-phase procedure
Both MALDI and LDI mass spectra of Pd1Au24(SR)18were acquired to gain insight into the skeletal structure of the cluster by studying the pattern of fragments.The mass peak patterns of Pd1Au24(SR)18and Au25(SR)18are indeed quite similar(Fig.4) in both MALDI and LDI.In the MALDI spectra of Pd1Au24(SR)18(Fig.4(A),upper panel),when the pulsed laser intensity(337 nm,nanosecond N2laser)was sufficiently intense,a fragment at m/z~5964 was observed,which is assigned to Pd1Au20(SR)14(theoretical m/z:5966.5).The loss of Au4(SC2H4Ph)4is identical to the case of homogold Au25(SR)18.In the latter,a fragment at m/z 6064(assigned to Au21(SR)14,theoretical m/z:6057.2) was found in the MALDI spectrum of Au25(SR)18.45,49At present, it has not been clear to us how Au25(SR)18and Pd1Au24(SR)18lose the Au4(SR)4unit.However,according to the core-shell structure of Au25(SR)18,it is most plausible that the Au4(SR)4unit is lost from the Au2(SR)3staple motifs,instead of involving the Au13core.The observation of a similar loss of Au4(SR)4unit in both Pd1Au24(SR)18and Au25(SR)18clusters strongly suggests that they have a similar skeletal structure and that the palladium atom should be located in the icosahedral core.If the Pd atom were located in one of the Au2(SR)3staples,one would have observed a Pd1Au3(SR)4fragment.
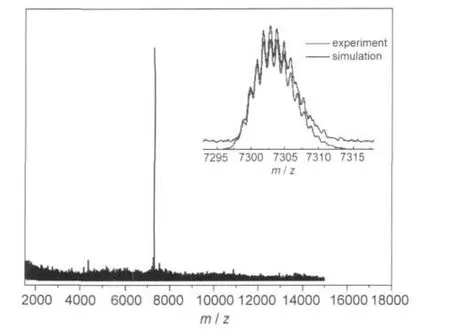
Fig.3 High resolution MALDI mass spectrum of isolated Pd1Au24(SC2H4Ph)18clusters(collected in reflection mode)Inset shows the isotope pattern of m/z 7303,together with a simulated pattern based on a formula Pd1Au24S18C144H162and a resolving power of 8000.
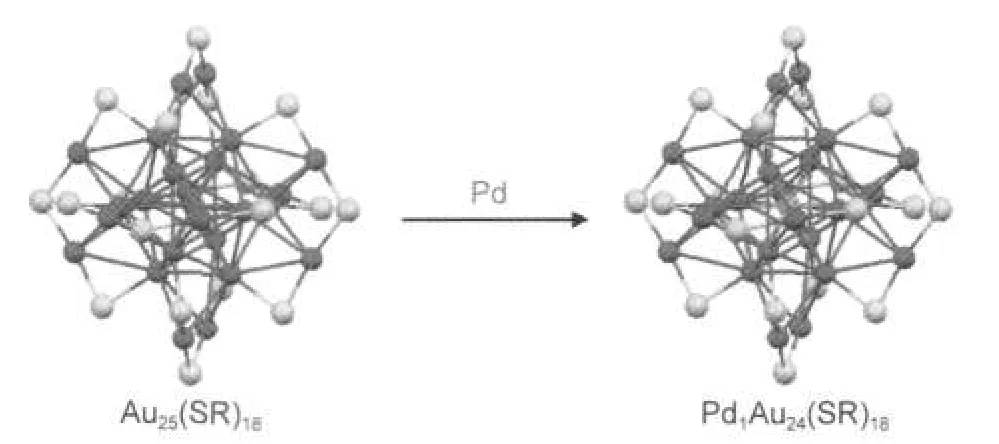
Scheme 1 Central doping of Pd in the 25-atom cluster
The LDI spectra(Fig.4(B))of the two clusters also demonstrate that they have a similar framework structure.The major peak atm/z 5213 from Pd1Au24(SR)18corresponds to [Pd1Au24S12]-(theoretical m/z:5218).Likewise,the most abundant peak in the LDI mass spectra of Au25(SR)18is[Au25S12]-at m/z 5305.25,49Recently,Wu et al.49interpreted the formation pathway of[Au25S12]-through the process:Au25(SR)18→[Au25S12]-+6S+18R.The similar fragments,[Pd1Au24S12]-vs [Au25S12]-,strongly imply that both Pd1Au24(SR)18and Au25(SR)18clusters should have a similar structure.It is noted that the structure of[Au25S12]-fragment has been theoretically computed by Jiang et al.53The[Au25S12]-ion should have a symmetric core-in-cage structure:a metallic Au core inside a Au-S cage. [Pd1Au24S12]-should have a similar core-in-cage structure.
3.3 Optical absorption properties of Pd1Au24(SR)18clusters
Fig.5 shows the UV-Vis absorption spectra of Pd1Au24(SR)18and[Au25(SR)18]-clusters.Due to strong quantum size effects, Pd1Au24(SR)18and[Au25(SR)18]-clusters show similar multiple transitions in the optical spectra.Although there is only one atom difference between the two clusters,their UV-Vis spectra indeed exhibit distinct differences.The spectrum of the Pd1Au24(SR)18clusters(Fig.5(A,B),wavelength and energy scales,respectively)is also compared with dodecanethiolate protected Pd1Au24clusters reported by Negishi et al.28Both types of clusters show peaks at 3.28,2.77,2.47,and 1.88 eV,indicating that the Pd1Au24core,rather than the type of thiolate,determines the optical properties.By comparing the experimental spectrum with theoretical ones computed by DFT,28the Pd1Au24(SR)18cluster with the Pd atom at the central position matches well with the experimental spectrum,thus,the optical spectrum provides another evidence for the Pd-doped core-shell structure of Pd1Au24(SR)18.As for Au25clusters,the optical absorbance spectrum shows multiple bands at 3.10,2.75,1.80,and 1.55 eV (Fig.5(C,D)).The HOMO-LUMO gap of Pd1Au24(SR)18was determined to be 1.40 eV,slightly higher than the~1.30 eV gap of Au25clusters.Both gap values are consistent with DFT calculations;Jiang et al.32predicted by DFT calculations that the Pd1@Au24(SR)18should have a larger HOMO-LUMO gap thanAu1@Au24(SR)18.
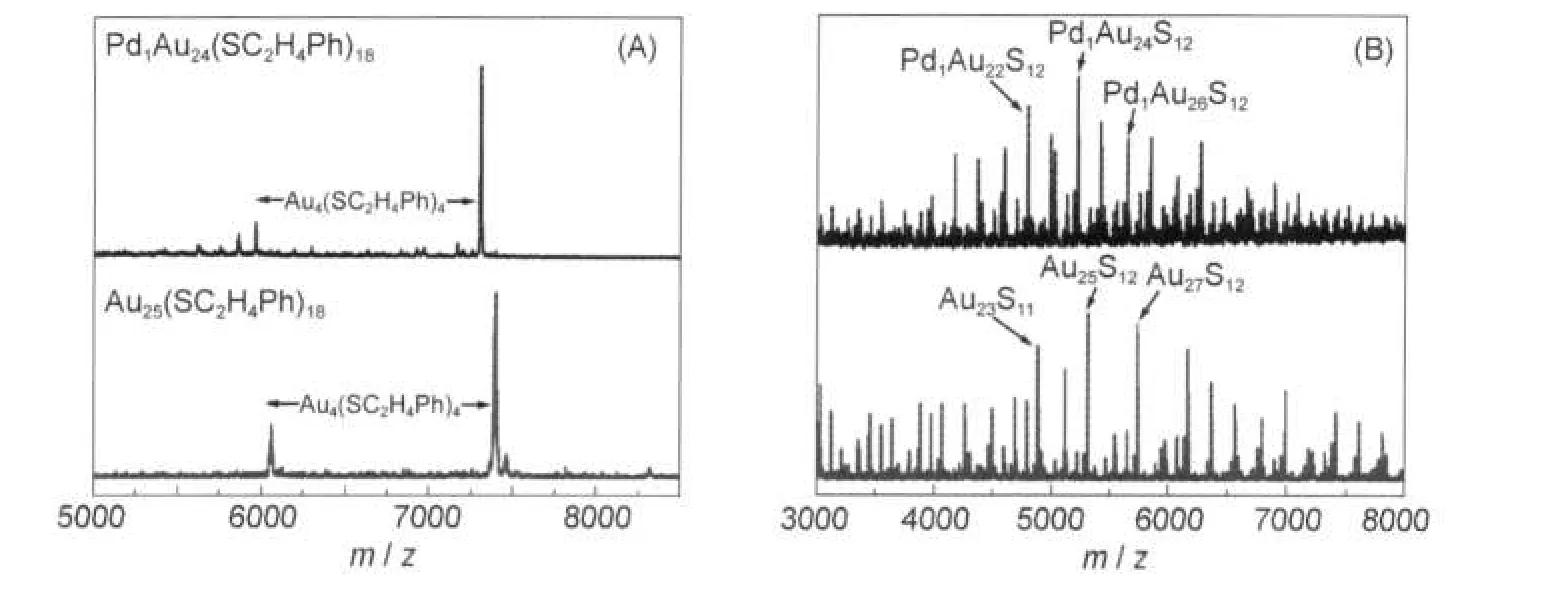
Fig.4 (A)Negative mode MALDI mass spectra of Pd1Au24(SR)18(top)andAu25(SR)18(bottom), (B)Negative mode LDI mass spectra of Pd1Au24(SR)18(top)andAu25(SR)18(bottom)
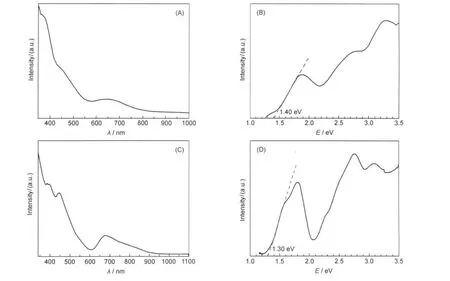
Fig.5 UV-Vis spectra of Pd1Au24(SR)18clusters based on the wavelength(A)and energy(B)scales,and spectra of[Au25(SR)18]-clusters based on the wavelength(C)and energy(D)scalesEnergy-dependent absorbance(Abs(E))vs pwavelength-dependent absorbance(Abs(λ)).Abs(E)∝Abs(λ)×λ2.
Taken together,these experimental and theoretical results all confirm that Pd1Au24(SR)18should adopt a core-shell structure comprising a Pd-doped icosahedral core(Pd1@Au12)and a homogoldAu12shell.
3.4 Doping of Au38(SR)24clusters with Pd
Interestingly,we also found similar central doping behavior in another cluster,Au38(SR)24.Au38(SR)24is also a very stable cluster.12,37,38We have recently determined its crystal structure by X-ray crystallography.12The cluster turned out to be chiral and the unit cell comprises two enantiomers(left-handed and right-handed).The cluster is composed of a face-shared biicosahedralAu23core and anAu15(SR)24shell.
Unlike Au25(SR)18,Au38(SR)24has two icosahedral centers.If the palladium atom is doped in the center of either icosahedron,one expects to observe Pd1Au37(SR)24and Pd2Au36(SR)24in the product.This is indeed what we have observed as side products in the two-phase synthesis of Pd-doped 25-atom nanoclusters(Fig.6).In the crude product(a mixture comprising both 25-atom and 38-atom clusters)prepared by the two-phase procedure,two small peaks at m/z~10602 and 10691 were found and assigned to Pd2Au36(SR)24(theoretical m/z:10597) and Pd1Au37(SR)24(theoretical m/z:10687).The small deviation is due to the gross calibration of the mass spectrometer.The low yield of Pd1Au37(SR)24and Pd2Au36(SR)24renders it not feasible to isolate them by SEC at this point.But the observation of mono-Pd and di-Pd doped 38-atom clusters provides another evidence that,in both 25-atom and 38-atom clusters,the palladium atom should be at the center of the icosahedron.
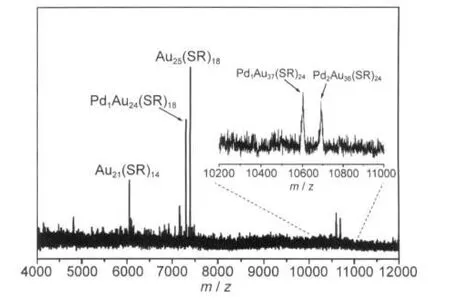
Fig.6 Negative-mode MALDI mass spectra ofAuPd clusters synthesized by two-phase procedureInset shows zooming in the mass range from m/z~10200 to 11000.
3.5 Catalytic activity of Pd1Au24(SR)18clusters
Gold nanoparticles(including nanoclusters and nanocrystals)show remarkable catalytic activity in many oxidization and hydrogenation reactions.20-23However,in the case of conventional nanoparticle catalysts,the polydispersity and unknown surface structure of Au nanoparticles preclude an in-depth understanding of the nature of catalysts and correlation of the structure and catalytic properties.In our recent work,we found that intact Au25(SR)18nanoclusters catalyzed hydrogenation of α,β-unsaturated ketones and aldehydes to unsaturated alcohols with 100%selectivity.23The electron-rich Au13core and the low-coordinate surface Au atoms in the Au12shell in the Au25(SR)18cluster are responsible for the remarkable catalytic performance observed.23Herein,we test the catalytic activity of pure Pd1Au24(SR)18isolated by SEC.The hydrogentation reaction of α,β-unsaturated ketones(e.g.,benzalacetone)by H2is chosen as a probe reaction for this purpose. Pd1Au24(SR)18clusters supported on iron oxide shows a catalytic activity comparable to that of Au25(SR)18/Fe2O3(42%conversion vs 40%).The similar catalytic activity of Pd1Au24(SR)18and Au25(SR)18implies that these two clusters have a similar structure and that the central palladium atom does not significantly affect the catalytic properties since the central atom is not directly exposed to reactants,nor involved in the surface reaction.
4 Conclusions
In summary,this work reports two new procedures to synthesize palladium doped 25-atom and 38-atom gold nanoclusters. Monodisperse Pd1Au24(SR)18nanoclusters are isolated from Au25(SR)18by size exclusion chromatography.On the basis of the similar fragments of the two clusters observed in MALDI and LDI mass spectra,the information from the UV-Vis spectra,and other evidences as discussed,we conclude that the mono-palladium dopant is located in the center of the 25-atom structure.The central doping behavior is also observed in the case of Au38(SR)24nanoclusters,including mono-Pd and di-Pd doped clusters,as the 38-atom cluster possesses a biicosahedral core.The as-obtained Pd1Au24(SR)18nanoclusters are found to catalyze the selective hydrogenation of α,β-unsaturated ketone to unsaturated alcohol with a conversion of 42%. The similar catalytic activity of Pd1Au24(SR)18and Au25(SR)18also supports that these two clusters have a similar core-shell structure.
(1) Jin,R.Nanoscale 2010,2,343.
(2) Schaaff,T.G.;Knight,G.;Shafigullin,M.N.;Borkman,R.F.; Whetten,R.L.J.Phys.Chem.B 1998,102,10643.
(3) Parker,J.F.;Fields-Zinna,C.A.;Murray,R.W.Accounts Chem. Res.2010,43,1289.
(4) Tsunoyama,H.;Tsukuda,T.J.Am.Chem.Soc.2009,131, 18216.
(5) Zhu,Y.;Wu,Z.;Gayathri,G.C.;Qian,H.;Gil,R.R.;Jin,R. J.Catal.2010,271,155.
(6) Li,J.;Wang,S.G.J.Mol.Model.2010,16,505.
(7) Shichibu,Y.;Negishi,Y.;Watanabe,T.;Chaki,N.K.; Kawaguchi,H.;Tsukuda,T.J.Phys.Chem.C 2007,111,7845.
(8) Jiang,D.E.Acta Phys.-Chim.Sin.2010,26,999.
(9) Zhou,R.J.;Shi,M.M.;Chen,X.Q.;Wang,M.;Chen,H.Z. Chem.-Eur.J.2009,15,4944.
(10) Zhu,M.;Aikens,C.M.;Hollander,F.J.;Schatz,G.C.;Jin,R. J.Am.Chem.Soc.2008,130,5883.
(11) Zhu,M.;Eckenhoff,W.T.;Pintauer,T.;Jin,R.J.Phys.Chem.C 2008,112,14221.
(12) Qian,H.;Eckenhoff,W.T.;Zhu,Y.;Pintauer,T.;Jin,R.J.Am. Chem.Soc.2010,132,8280.
(13) Schaaff,T.G.;Whetten,R.L.J.Phys.Chem.B 2000,104,2630. (14) Negishi,Y.;Nobusada,K.;Tsukuda,T.J.Am.Chem.Soc.2005, 127,5261.
(15) Wu,Z.;Jin,R.Nano Lett.2010,10,2568.
(16) Rao,T.U.B.;Pradeep,T.Angew.Chem.Int.Edit.2010,49, 3925.
(17)Zhu,M.;Aikens,C.M.;Hendrich,M.P.;Gupta,R.;Qian,H.; Schatz,G.C.;Jin,R.J.Am.Chem.Soc.2009,131,2490.
(18)Negishi,Y.;Tsunoyama,H.;Suzuki,M.;Kawamura,N.; Matsushita,M.M.;Maruyama,K.;Sugawara,K.;Yokoyama, T.;Tsukuda.T.J.Am.Chem.Soc.2006,128,12034.
(19) Iwasa,T.;Nobusada,K.Chem.Phys.Lett.2007,441,268.
(20) Tsunoyama,H.;Ichikuni,N.;Sakurai,H.;Tsukuda,T.J.Am. Chem.Soc.2009,131,7086.
(21) Zhu,Y.;Qian,H.;Zhu,M.;Jin,R.Adv.Mater.2010,22,1915.
(22) Zhu,Y.;Qian,H.;Jin,R.Chem.-Eur.J.2010,16,11455.
(23) Zhu,Y.;Qian,H.;Drake,B.A.;Jin,R.Angew.Chem.Int.Edit. 2010,49,1295.
(24) Jin,R.;Qian,H.;Wu,Z.;Zhu,Y.;Zhu,M.;Mohanty,A.;Garg, N.J.Phys.Chem.Lett.2010,1,2903.
(25) Zhu,M.;Lanni,E.;Garg,N.;Bier,M.E.;Jin,R.J.Am.Chem. Soc.2008,130,1138.
(26) Wu,Z.;Suhan,J.;Jin,R.J.Mater.Chem.2009,19,622.
(27) Parker,J.F.;Weaver,J.E.F.;McCallum,F.;Fields-Zinna,C. A.;Murray,R.W.Langmuir 2010,26,13650.
(28) Negishi,Y.;Kurashige,W.;Niihori,Y.;Iwasa,T.;Nobusada,K. Phys.Chem.Chem.Phys.2010,12,6219.
(29) Jiang,D.;Dai,S.Inorg.Chem.2009,48,2720.
(30) Kacprzak,K.A.;Lehtovaara,L.;Akola,J.;Lopez-Acevedoa, O.;Hakkinen,H.Phys.Chem.Chem.Phys.2009,11,7123.
(31)Walter,M.;Moseler,M.J.Phys.Chem.C 2009,113,15834.
(32) Jiang,D.E;Whetten,R.L.Phys.Rev.B 2009,80,115402.
(33)Akola,J.;Kacprzak,K.A.;Lopez-Acevedo,O.;Walter,M.; Gronbeck,H.;Hakkinen,H.J.Phys.Chem.C 2010,114,15986.
(34) Reveles,J.U.;Clayborne,P.A.;Reber,A.C.;Khanna,S.N.; Pradhan,K.;Sen,P.;Pederson,M.R.Nat.Chem.2009,1,310.
(35)Wyrwas,R.B.;Alvarez,M.M.;Khoury,J.T.;Price,R.C.; Schaaff,T.G.;Whetten,R.L.Eur.Phys.J.D 2007,43,91.
(36) Schaaff,T.G.;Shafigullin,M.N.;Khoury,J.T.;Vezmar,I.; Whetten,R.L.;Cullen,W.G.;First,P.N.;Gutierrez-Wing,C.; Ascensio,J.;Jose-Yacaman,M.J.J.Phys.Chem.B 1997,101, 7885.
(37) Chaki,N.K.;Negishi,Y.;Tsunoyama,H.;Shichibu,Y.; Tsukuda,T.J.Am.Chem.Soc.2008,130,8608.
(38) Qian,H.;Zhu,M.;Andersen,U.N.;Jin,R.J.Phys.Chem.A 2009,113,4281.
(39) Qian,H.;Zhu,Y.;Jin,R.J.Am.Chem.Soc.2010,132,4583.
(40) Fields-Zinna,C.A.;Crowe,M.C.;Dass,A.;Weaver,J.E.F.; Murray,R.W.Langmuir 2009,25,7704.
(41) Qian,H.;Jin,R.Nano Lett.2009,9,4083.
(42) Dharmaratne,A.C.;Krick,T.;Dass,A.J.Am.Chem.Soc.2009, 131,13604.
(43) Zhu,M.;Qian,H.;Jin,R.J.Phys.Chem.Lett.2010,1,1003.
(44)Wu,Z.;Lanni,E.;Chen,W.;Bier,M.E.;Ly,D.;Jin,R.J.Am. Chem.Soc.2009,131,16672.
(45) Dass,A.;Stevenson,A.;Dubay,G.R.;Tracy,J.B.;Murray,R. W.J.Am.Chem.Soc.2008,130,5940.
(46)Xiang,H.J.;Wei,S.H.;Gong,X.G.J.Am.Chem.Soc.2010, 132,7355.
(47) MacDonald,M.A.;Zhang,P.;Qian,H.;Jin,R.J.Phys.Chem. Lett.2010,1,1821.
(48)Angel,L.A.;Majors,L.T.;Dharmaratne,A.C.;Dass,A.ACS Nano 2010,4,4691.
(49) Wu,Z.;Gayathri,C.;Gil,R.;Jin,R.J.Am.Chem.Soc.2009, 131,6535.
(50) Kogo,A.;Sakai,N.;Tatsuma,T.Electrochem.Commun.2010, 12,996.
(51)Zheng,N.;Johnson,J.P.;Williams,C.C.;Wang,G. Nanotechnology 2010,21,295708.
(52)Wang,Z.;Cai,W.;Sui,J.ChemPhysChem 2009,10,2012.
(53) Jiang,D.E.;Walter,M.;Dai,S.Chem.-Eur.J.2010,16,4999.
