苯基取代聚苯撑乙烯的合成及其电致发光性能
2011-11-30莫越奇常学义胡苏军韩绍虎吴宏滨彭俊彪
莫越奇 常学义 胡苏军 韩绍虎 吴宏滨,* 彭俊彪
(1华南理工大学材料科学与工程学院,广州510640;2广州砺剑光电材料科技有限公司,广州510730; 3华南理工大学高分子光电材料及器件研究所,广州510640)
苯基取代聚苯撑乙烯的合成及其电致发光性能
莫越奇1,*常学义2胡苏军3韩绍虎3吴宏滨3,*彭俊彪3
(1华南理工大学材料科学与工程学院,广州510640;2广州砺剑光电材料科技有限公司,广州510730;3华南理工大学高分子光电材料及器件研究所,广州510640)
聚苯撑乙烯(PPV)类聚合物是优异的发光材料,有望作为全色显示中三基色的材料之一得到应用.我们采用2-溴-1,4-亚二甲苯二乙酯为原料,合成了商品名为Supper Yellow PPV(SY PPV)的苯基取代PPV.中间体、单体和聚合物的结构都通过核磁共振、元素分析进行了表征.SY PPV的吸收峰在434 nm,吸收边在510 nm,带隙2.44 eV.光致发光峰值和电致发光峰值分别在516和552 nm.SY PPV的器件性能为:启动电压为2.4 V,最大亮度大于49000 cd·m-2,最大流明效率为21 cd·A-1,显著优于采用老方法合成SY PPV的最大流明效率(16-18 cd·A-1).
聚苯撑乙烯;苯基取代聚苯撑乙烯;绿色发光聚合物;聚合物发光二极管;电致发光
1 Introduction
Poly(phenylenevinylene)s(PPVs)are one of the most important materials for polymer light-emitting diode(PLED)applications.Although blue emission is unavailable from PPVs,one of the phenyl-substituted PPVs1with trade mark name of“Super Yellow PPV”(SY PPV)is the most efficient green light emitters to date and had been used in the first commercial PLED displays such as the Philips shaver and“magic mirror”mobile telephone.
It should be pointed out that the chemical structures of phenyl-substituted PPVs determine their device performance.SY PPV is a copolymer containing 2-alkyloxyphenyl-1,4-phenylenevinylene derivatives,and it was reported that device from SY PPV exhibits a high luminous efficiency(LE)of 14.8 cd· A-1.1With effort of incorporating an electron injection layer into the devices,we were able to improve the LE to 23.8 cd·A-1in 2004.2However,modification on the chemical structure of SY PPVs usually does not lead to improvement of device performance.For example,when phenyl or alkyl/alkyloxy groups was introduced to the 3,5,6-position of phenylene units in SY PPV,it had been demonstrated that poly(2,3-diphenyl-1,4-phenylenevinylene)s3,4only showed moderate device performance and only recently devices based on their copolymers showed a LE of 6.14 cd·A-1.5Furthermore,it is worthy to note that poly(2-phenyl-5-alkyloxy-1,4-phenylenevinylene)only exhibits an external quantum efficiency(EQE)of 0.01%;6despite that the lifetime can be improved by introducing 2-phenyl-5-methoxy-1,4-phenylenevinylene units into SY PPV.7Although poly(2,5-diphenyl-1,4-phenylenevinylene)s8and poly(2,3,6-triphenyl-1,4-phenylenevinylene)s9were also synthesized,the study on their EL properties rarely reported.Another strategy is to incorporate other aryl groups instead of alkyloxyphenyl on the 2-position of phenylene units in SY PPV.Chen et al.10reported the synthesis of poly(2-biphenyl-1,4-phenylenevinylene)derivatives and found that PLEDs from the obtained polymer only showed an EQE of 0.66%.In addition,it had been demonstrated that PPV derivatives bearing highly phenylated side groups,11fluorene side groups,12,132-spirofluorene,142-anthracene,15and 2-stilbene16side groups also exhibited low efficiency.An alternative method is to modify the solubilizing substituents on 2-phenyl of SY PPV.It is well known that 4-(3′,7′-dimethyoctyloxy)and/or 3-(3′,7′-dimethyoctyloxy)are typical solubilizing substituents on 2-phenyl of SY PPV.Johansson et al.17,18synthesized various poly(2-dialkyoxylphenyl-1,4-phenylenevinylene)derivatives and got an EQE of 1.15%although these polymers have better solubilities.A silyl solubilizing substituents such as poly(2-(3′-dimethyldodecylsily)lphenyl-1,4-phenylenevinylene)exhibited a power efficiency of 0.25 lm·W-1.19,20
As mentioned above,the chemical modification did not show significant improvement in device performance,however,it was found that the polymerization conditions21during the synthesis of PPV can have a large impact on its EL properties. Aiming at highly efficient polymer for PLEDs,we carry research on the study of optimized synthesis of SY PPV,and here we report a new route to prepare the monomers of SY PPV,which eventually leads to efficient PLEDs with luminous efficiency over 21 cd·A-1thus finds applications in full-color flat panel displays or solid state lighting sources.
2 Experimental
2.1 Characterization
NMR(400 MHz)spectra were obtained using a Bruker 400 MHz spectrometer with tetramethylsilane as an internal standard.C,H,N elemental analyses were performed on a Vario EL elemental analysis instrument(Elementar Co.)and Cl elemental content was analyzed by chemical titration.Gel Permeation Chromatography(GPC)analysis was conducted with a Waters GPC 2410 in tetrahydrofuran(THF)using a calibration curve of polystyrene standards.UV-visible absorption spectra were recorded on a HP 8453 UV-Vis spectrophotometer.The photoluminescent(PL)spectra were obtained with a Fluorolog JY luminescence spectrometer.Cyclic voltammetry was carried out on a Potentiostat/Galvanostat Model 283 (Princeton Applied Reserch Co.)in a solution of tetrabutylammonium hexafluorophosphate(Bu4NPF6)(0.1 mol·L-1)in acetonitrile at a scanning rate of 50 mV·s-1at room temperature under the protection of argon.
2.2 Materials
1-Bromo-2,5-bis(bromomethyl)benzene was prepared according to the literature.224-(3′,7′-dimethyoctyloxy)phenylboronic acid,3-(3′,7′-dimethyoctyloxy)phenylboronic acid,and 2-methoxy-5-(2′-ethylhexyloxy)-1,4-bis(methylchloride)benzene was obtained from Canton Oledking Optoelectronic Materials Co.,Ltd.Other chemicals and reagents were used as received from Aldrich,TCI,and Acros Chemical Co.unless specified otherwise.All solvents were carefully dried and purified before use.All manipulations involving air-sensitive reagents were performed under a dry argon atmosphere.
2.2.1 2-Bromo-1,4-xylylene diacetate(1)
A mixture of 34.3 g of 1-bromo-2,5-bis(bromomethyl)benzene(0.1 mol),24.8 g of sodium actate(0.3 mol)in 200 mL of acetic acid were refluxed over night.The solvent was stripped off under vacuum and the residue was cooled to room temperature to afford yellowish solid.After washed with brine for several times and filtered,the crude product was purified by recrystallization from ethanol to afford a white solid 1(Tm= 66-68°C,yield:24.6 g,82%).Anal.Calcd.for C12H13BrO4(%):C,47.86;H,4.35;Br,26.53.Found(%):C,47.82;H, 4.32;Br,26.46.1H NMR(400 MHz,CDCl3):7.57(s,1H),7.38 (d,J=8.0,1H),7.28(d,J=8.0,1H),5.16(s,2H),5.04(s,2H), 2.12(m,3H),2.10(m,3H).13C NMR(100 MHz,CDCl3): 170.63,170.57,137.85,135.11,132.29,129.85,127.15, 123.39,65.49,64.87,20.92,20.86.
2.2.2 2-(4-(3′,7′-Dimethyloctyloxy))phenyl-1,4-xylylene diacetate(2)
A mixture of compound 1(9 g,0.03 mmol),4-(3′,7′-dimeth-yloctyloxy)phenylboronic acid(13.85 g,0.05 mol),tetrakis(triphenylphosphine)palladium(0.115 g,0.1 mmol),aliquat 336 (0.2 g)and aqueous sodium carbonate(2 mol·L-1,0.2 mol)in 100 mL toluene were stirred in nitrogen at 90°C for 2 days and cooled to room temperature.The reaction mixture was washed with brine for several times,and the organic layers were dried over MgSO4and concentrated.The crude product was purified by vacuum distillation,resulting colorless oil (yield:11.7 g,86%).Anal.Calcd.for C28H37O5(%):C,73.98; H,8.43.Found(%):C,73.91;H,8.40.1H NMR(400 MHz, CDCl3):7.48(d,J=8.0,1H),7.37-7.28(m,3H),6.91-6.87 (m,3H),5.13(s,2H),5.03(s,2H),4.01(m,2H),2.10(s,3H), 2.05(s,3H),1.83(m,1H),1.68-1.50(m,3H),1.34-1.14(m, 6H),0.94-0.93(m,3H),0.86-0.85(m,6H).13C NMR(100 MHz,CDCl3):170.90,170.72,158.92,142.56,141.18,135.96, 133.20,129.81,129.78,129.26,127.29,121.26,115.28, 113.64,66.29,65.82,64.11,39.22,37.29,36.21,29.84,29.81, 27.97,24.67,22.72.
2.2.3 2-(4-(3′,7′-Dimethyloctyloxy))phenyl-1,4-xylylenediol(3)
To a solution of LiAlH4(3.45 g,0.1 mol)in 150 mL of tetrahydrofuran was added dropwise a solution of compound 2 (3.45 g,0.1 mol)in 90 mL of tetrahydrofuran.The mixture was stirred at room temperature for 6 h and quenched carefully with 145 mL of 5%hydrochloric acid.The organic layer was separated,the aqueous layer was extracted with ether for five times,and the combined organic layer dried over Na2SO4and concentrated.The crude product was purified by silica gel chromatography using 30%ethyl acetate in hexane to give a white solid(yield:28.1 g,69%).Anal.Calcd.for C24H34O3(%):C, 77.80;H,9.25.Found(%):C,77.72;H,9.26.1H NMR(400 MHz,CDCl3):7.49(d,J=7.6,1H),7.34-7.25(m,3H), 6.91-6.88(m,3H),4.68(s,2H),4.59(s,2H),4.00(m,2H), 2.29(s,2H),1.83(m,1H),1.69-1.52(m,3H),1.35-1.16(m, 6H),0.95-0.93(m,3H),0.88-0.86(m,6H),2.29(s,2H),1.83 (m,1H),1.69-1.52(m,3H),1.35-1.16(m,6H),0.95-0.93 (m,3H),0.88-0.86(m,6H).13C NMR(100 MHz,CDCl3): 158.93,141.77,141.29,140.17,137.32,129.24,128.63, 128.45,126.20,121.28,115.28,113.42,66.37,64.85,62.82, 39.24,37.31,36.22,29.85,27.99,24.68,22.74,22.63,19.67.
2.2.4 2-(3-(3′,7′-Dimethyloctyloxy)phenyl)-1,4-bis(methylchloride)benzene(4)
To a solution of 2-(4-(3′,7′-dimethyloctyloxy))phenyl-1, 4-xylylenediol(37 g,0.1 mol)in 150 mL of toluene was added dropwise 25 mL of SOCl2(0.33 mol).The dark mixture was stirred at room temperature for 4 h and quenched carefully with 100 mL of water.The organic layer was separated,the aqueous layer was extracted with ether for five times,and the combined organic layer dried over Na2CO3and concentrated. The crude product was purified by silica gel chromatography using 10%ethyl acetate in hexane to give a pale greenish oil (yield:37.8 g,93%).Anal.Calcd.for C24H32Cl2O(%):C, 70.75;H,7.92;Cl,17.40.Found(%):C,70.72;H,7.91;Cl, 17.36.1H NMR(400 MHz,CDCl3):7.54(d,J=8.0,1H),7.41 (d,J=8.0,1H),7.36-7.33(m,2H),6.98-6.93(m,3H),4.60(s, 2H),4.53(s,2H),4.05(m,2H),1.85(m,1H),1.72-1.55(m, 3H),1.36-1.19(m,6H),0.98(m,3H),0.90(m,6H).13C NMR (100 MHz,CDCl3):159.00,142.41,140.83,137.70,135.08, 131.04,130.32,129.42,128.06,121.22,114.96,114.17,66.4, 45.65,44.04,39.24,37.30,36.20,29.86,28.0,24.70,22.75, 22.65,19.70.
2.2.5 2-(4-(3′,7′-Dimethyloctyloxy))phenyl-1,4-xylylene diace-tate(5)
Compound 5 was prepared as a colorless oil(yield:87%, started from compound 1 and 3-(3′,7′-dimethyloctyloxy) phenylboronic acid)according to the similar synthetic route of compound 2.Anal.Calcd.for C28H37O5(%):C,73.98;H,8.43. Found(%):C,73.91;H,8.40.1H NMR(400 MHz,CDCl3): 7.48(d,J=6.8,1H),7.34-7.23(m,4H),6.95(d,J=6.8,2H), 5.12(s,2H),5.03(s,2H),4.03(m,2H),2.10(s,3H),2.06(s, 3H),1.85(m,1H),1.70-1.51(m,3H),1.34-1.17(m,6H), 0.96(m,3H),0.87(m,6H).
2.2.6 2-(4-(3′,7′-Dimethyloctyloxy))phenyl-1,4-xylylenediol(6)
Compound 6 was prepared as a colorless oil(yield:84%), started from compound 5 and LiAlH4according to the similar synthetic route of compound 3.Anal.Calcd.for C24H34O3(%): C,77.80;H,9.25.Found(%):C,77.72;H,9.26.1H NMR(400 MHz,CDCl3):7.45(d,J=7.6,1H),7.28-7.22(m,4H),6.92(d, J=7.6,2H),4.64(s,2H),4.56(s,2H),4.01(m,2H),1.84(m, 1H),2.37(s,2H),1.69-1.53(m,3H),1.37-1.18(m,6H), 0.97-0.96(m,3H),0.89-0.88(m,6H).13C NMR(100 MHz, CDCl3):158.44,141.09,140.2,137.35,132.54,130.15,128.7, 125.83,114.22,66.37,64.81,62.86,39.26,37.32,36.23, 29.86,28.00,24.68,22.77,22.66,19.69.13C NMR(100 MHz, CDCl3):170.84,170.71,158.64,142.37,135.96,133.31, 131.98,130.15,130.06,129.83,126.95,114.24,66.31,65.85, 64.18,39.24,37.28,36.20,29.83,27.99,24.68,22.74,22.63, 21.02,19.68.
2.2.7 2-(4-(3′,7′-Dimethyloctyloxy)phenyl)-1,4-bis(methylchlo-ride)benzene(7)
Compound 7 was prepared as a pale greenish oil(yield: 85%)according to the similar synthetic route of compound 4. Anal.Calcd.for C24H32Cl2O(%):C,70.75;H,7.92;Cl,17.40. Found(%):C,70.72;H,7.91;Cl,17.36.1H NMR(400 MHz, CDCl3):7.54(d,J=8.0,1H),7.39(d,J=8.0,1H),7.35(d,J= 8.0,2H),7.31(s,1H),6.99(d,J=8.0,2H),4.61(s,2H),4.54 (s,2H),4.05(m,2H),1.87(m,1H),1.72-1.54(m,3H), 1.38-1.19(m,6H),0.99-0.97(m,3H),0.90-0.89(m,6H).13C NMR(100 MHz,CDCl3):158.77,142.23,137.67,135.19, 131.64,131.02,130.58,130.17,127.68,114.34,66.36,45.69, 44.16,39.25,37.29,36.20,29.85,28.0,24.69,22.75,22.64, 19.69.
2.2.8 SY PPV
A solution of 2.0 g of compound 4(4.9 mmol),2.0 g of 7 (4.9 mmol)and 66 mg of 2-methoxy-5-(2′-ethylhexyloxy)-1, 4-bismethychlorobenzene(0.2 mmol)in 300 mL of dioxane was stirred mechanically at 97°C under N2flow,and 4.48 g of t-BuOK(40 mmol)dissolved in 50 mL of dioxane was added in 0.5 min.After refluxing for 2 h,the mixture was cooled down and 350 mL ethanol/water solution(1:1)was added to precipitate for the first time.The precipitation was collected and washed with water and ethanol for 3 times,dried at 50°C for 12 h under vacuum to afford 2.0 g of crude product as a golden powder.The crude product was dissolved in 200 mL of xylene and put through G2(30-50 μm)filter to remove the insoluble gel.The filtrate was precipitated in 400 mL of ethanol for the second time to remove the oligomers.The precipitation was collected and dried at 50°C for 12 h under vacuum to afford 1.5 g of SY PPV as a golden fiber(yield:45%).1H NMR (400 MHz,CDCl3):7.7-6.9(m,18H),4.60(s,2H),4.03(m, 4H),1.8-0.9(m,38H).
2.3 Device fabrication and characterization
SY PPV was dissolved in toluene and filtered through a 0.45 μm PTFE filter before use.Patterned indium tin oxide(ITO) coated glass with a sheet resistance of 15-20 Ω·□-1were cleaned by a surfactant scrub,then underwent a wet-cleaning process inside an ultrasonic bath,beginning with deionized water,followed by acetone and isopropanol.After oxygen plasma cleaning for 4 min,a 100 nm-thick poly(3,4-ethylenedioxythiopene):poly(styrenesulfonate)(PEDOT:PSS)(Bayer Baytron 4083)anode buffer layer was spin-cast on the ITO substrate and then dried by baking in a vacuum oven at 80°C overnight. Then the solution of the polymers in toluene was spin coated on the top of the obtained substrates.The typical thickness of the emitting layer was 70-80 nm.Finally,3 nm barium and 100 nm aluminum layer were evaporated with a shadow mask at a base pressure of 3×10-4Pa.Except for the deposition of the PEDOT layers,all the fabrication processes were carried out inside a controlled atmosphere of nitrogen dry-box(Vacuum Atmosphere Co.)containing oxygen and moisture with volume fraction less than 10-5.The current density-luminance-voltage(J-L-V)characteristic was measured using a Keithley 236 source-measurement unit and a calibrated silicon photodiode.The forward-viewing luminance was calibrated by a spectrophotometer(SpectraScan PR-705,Photo Research)and the forward-viewing LE was calculated accordingly.Throughout the whole manuscript,reported value of luminance and LE are for forward-viewing direction only.The external quantum efficiency(EQE)of electroluminescence(EL)was collected by measuring the total light output in all directions in an integrating sphere(IS-080,Labsphere).The EL spectra were collected by a PR-705 photometer.
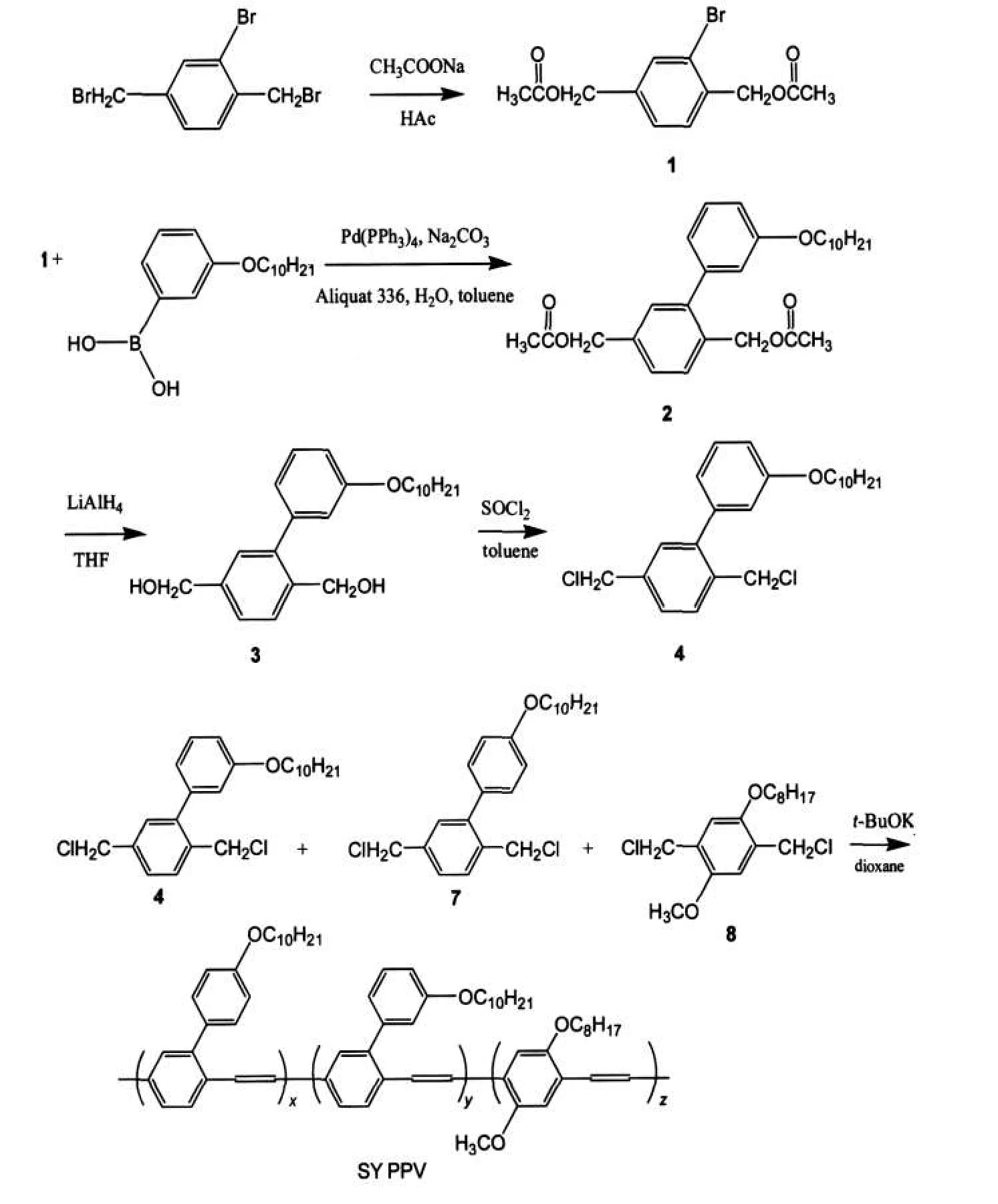
Scheme 1 Synthesis of SY PPV
3 Results and discussion
3.1 Synthesis of the polymer
The synthesis of SY PPV was plotted in Scheme 1.It should be pointed out that we prepared a novel intermediate—2-bromo-1, 4-xylylene diacetate(1)in this contribution.With compound 1 and corresponding phenylboronic acid,2-(3-(3′,7′-dimethyloctyloxy)phenyl)-1,4-xylylene diacetate(2)and 2-(4-(3′,7′-dimethyloctyloxy)phenyl)-1,4-xylylene diacetate(5)can be obtained via a typical Suzuki coupling reaction.Compound 2 and 5 can be reduced to the correspending 2-phenyl-1,4-xylylenediols(3 and 6)using LiAlH4as a reductant,followed by the reaction with SOCl2to afford the correspending monomers(4 and 7).Typically,SY PPV is polymerized with three monomers, herein we use 2-methoxy-5-(2′-ethylhexyloxy)-1,4-bismethychlorobenzene(8)besides 4 and 7.8 is very famous for that it is the momomer of MEH-PPV.23However,the molar feed ratio of 8 is only 2%in this copolymerization because it is reported that the increase content(>2%)of the 2,5-dialkyloxy-1,4-phenylenevinylene always led to the redshift of emission spectra of the resulting copolymers,together with the decrease of the EL efficiencies.1SY PPV is readily soluble in common organic solvents,including toluene,xylene,and THF.The numberaverage and weight-average molecular weights of SY PPV,as determined through gel permeation chromatography(GPC)using polystyrenes as standards,were 20×104and 80×104,respectively,with polydispersities of 4.0.
Generally,the commercially available 2-bromo terephthalic acid is esterified to afford 2-bromo terephthalate(9),which is used as the starting material,according to the literature(as shown in Scheme 2).1We found that it was difficult to get pure enough 9 by distillation because the boiling points of monoesters(10 and 11)are close to that of 9.Howover,we can easily realize mass production of pure enough 1 by recrystallation. That is why we exploited this approach.The resulting polymer exhited higher EL efficiency than that using 9 as starting material,which will be carefully demonstrated in the following paragraph.
It has been reported that diethyl 2-(3-(3′,7′-dimethyloctyloxy)phenyl)terephthalate(13)was synthesized via Suzuki coupling reaction with 2-bromo terephthalic acid used as the starting material,followed by the reaction with LiAlH4to afford compound 3(as shown in Scheme 2).After treated with SOCl2,we can get the monomer 4.Monomer 7 can be obtained under the same procedure with the corresponding reactants.Finally,we can get the resulting copolymer(SY PPV)with the monomers(4,7,8)via a modified Gilch reaction and its NMR spectrum was shown in Fig.1.We can find the peaks assigned to aryl and alkyl groups,especailly the peaks arround 2.7-2.9, which is assigned to tolane-bisbenzyl moieties(TBB)by Becker et al.24High content of TBB responds for the poor device stability,but have no great influence on the EL efficiency.In order to improve the device stability,Becker et al.7abandoned monomers such as 4 and 7 in 2002 because they resulted in high content of TBB moeities during the polymerization.Recently,the results from Fleissner et al.25implied that rather the residual halide defect than TBB defect has to be considered to respond for the poor device stability.As highly efficient SY PPV can be obtained from monomers 4 and 7,we go on with the study on this kind of SY PPV and the long lifetime devices with highly EL efficiency will be reported elsewhere.
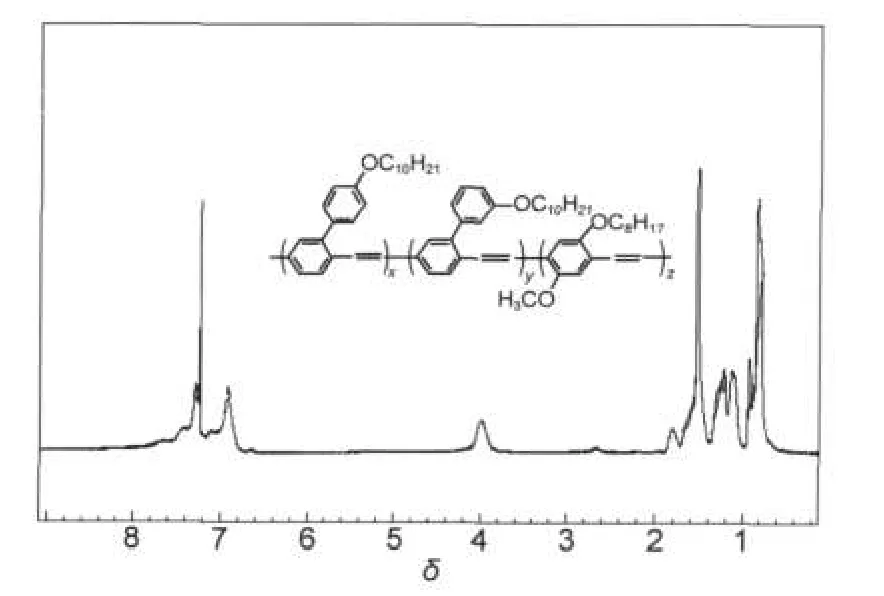
Fig.1 1H NMR spectrum of SY PPV
3.2 Photophysical properties of SY PPV
The absorption and PL/EL spectra of SY PPV are shown in Fig.2.It can be clearly seen that SY PPV film has absorption maximum at 434 nm and the absorption onset at 510 nm,thus the optical bandgap(Eg)of SY PPV is estimated at ca 2.44 eV.
The photoluminescence(PL)spectrum of SY PPV film were obtained with a Fluorolog JY luminescence spectrometer under excitation of 440 nm.As can be seen in Fig.2,the PL spectrum of SY PPV film shows a maximum at 516 nm with a shoulder at 546 nm,which means SY PPV emits yellowish green light.It was reported the copolymers obtained from monomers 4 and 7 emitted green light with CIE of(0.35, 0.61).1However,it was reported that with the addition of 2% molar feed ratio of dialkyloxyphenylenevinylene units,the obtained copolymers could exhibit higher electroluminescence EL efficiency.1Therefore,we adopt 2%molar feed ratio of dialkyloxyphenylenevinylen in this study to obtain yellowish green light-emitting polymer rather than the green light-emitting polymer.
3.3 Electrochemical characteristics of SY PPV
The electrochemical behavior of the polymers was investigated by cyclic voltammetry(CV).We can record only one pdoping process(shown in Fig.3).The onset potentials of the oxidation process(p-doping)occur at about 1.1 V,so the HOMO level of the SY PPV is-5.50 eV according to the empirical formula,EHOMO=-(Eox+4.4).26The LUMO levels were calculated to be about-3.06 eV from the HOMO level and the optical bandgap(Eg).Generally the electronic properties of PPVs derivative is typically hole-dominant,and characterized by much lower electron mobility,hence,exciton formation is usually limited by bottleneck of inefficient electron contribution.However, given the high-lying LUMO in the SY PPV studied here,Ohmic contact with low work function metal,such as Ba,Ca(with work function of-2.7-2.8 eV)would be expected,facilitating efficient electron injection,more balanced carriers transport injection and resulting in higher device efficiency.

Fig.2 Absorption,PL,and ELspectra of the SY PPV film

Fig.3 Cyclic voltammograms of SY PPV
3.4 Electroluminescent properties of the SY PPV
PLEDs with structure of ITO/PEDOT:PSS/emission layer/ Ba/Al was fabricated to investigate the electroluminescent properties of SY PPV.As can be seen from Fig.2,the EL spectrum of SY PPV device shows a slight red shift as compared with the PL spectrum.Furthermore,the intensity of long wavelength vibration increases,resulting in an EL maximum of 552 nm with a shoulder at 528 nm.The CIE coordinates of the obtained devices is calculated to be(0.388,0.578).
The current density-luminance-voltage characteristics(JL-V)of a SY PPV PLEDs are presented in Fig.4.The turn-on voltage(defined as the voltage at which a luminance of 1 cd· m-2was measured)of SY PPV is ca 2.4 V,while the luminance maximum exceeding 49000 cd·m-2was obtained at 8 V.The device exhibits a maximal luminous efficiency(LE)of 21 cd· A-1(corresponding to an EQE of 8.0%),much better than those EL efficiencies(typically of 16-18 cd·A-1)reported previously.1

Fig.4 Current density-luminance-voltagecharacteristics of SY PPV
We note that impurities in SY PPV may have big impact on the device performance,therefore,the significant improvement in the device performance can be attributed to the higher purity monomers obtained from our new synthesis approach.The investigation on the correlation between the EL efficiencies and the purity grade of the monomers is ongoing,and the results will be published in the forthcoming manuscript.
4 Conclusions
A new synthesis approach was put forward in this contribution.A new starting material,2-bromo-1,4-xylylene diacetate, with higher purity grade was prepared and the impurities of all the intermediates and monomers were strictly controlled.The resulting polymer exhibited a maximal luminous efficiency (LE)of 21 cd·A-1(corresponding to an external quantum efficiency(EQE)of 8.0%),which is the best device performance among SY PPV,reported to date.
(1) Spreitzer,H.;Becker,H.;Kluge,E.;Kreuder,W.;Schenk,H.; Demandt,R.;Schoo,H.Adv.Mater.1998,10,1340.
(2)Wu,H.B.;Huang,F.;Mo,Y.Q.;Yang,W.;Wang,D.L.;Peng, J.B.;Cao,Y.J.Soc.Inf.Display 2005,13,123.
(3)Hsieh,B.R.;Wan,W.C.;Yu,Y.;Gao,Y.;Goodwin,T.E.; Gonzalez,S.A.;Feld,W.A.Macromolecules 1998,31,631.
(4) Hsieh,B.R.;Yu,Y.;Forsythe,E.W.;Schaaf,G.M.;Feld,W.A. J.Am.Chem.Soc.1998,120,231.
(5) Chen,K.B.;Li,H.C.;Chen,C.K.;Yang,S.H.;Hsieh,B.R.; Hsu,C.S.Macromolecules 2005,38,8617.
(6) Boardman,F.H.;Grice,A.W.;Ruether,M.G.;Sheldon,T.J.; Bradley,D.D.C.;Burn,P.L.Macromolecules 1999,32,111.
(7) Becker,H.;Spreitzer,H.;Kreuder,W.;Kluge,E.;Schenk,H.; Parker,I.;Cao,Y.Adv.Mater.2000,12,42.
(8) Jin,Y.;Kim,J.;Park,S.H.;Kim,H.;Lee,K.;Suh,H.Bull. Korean Chem.Soc.2005,26,1807.
(9) Hsieh,B.R.;Yu,Y.Electroluminescent polymer compositions and processes thereof.U.S.Patent,5945502,1997.
(10)Chen,Z.K.;Lee,N.H.S.;Huang,W.;Xu,Y.S.;Cao,Y. Macromolecules 2003,36,1009.
(11) Mikroyannidis,J.A.Chem.Mater.2003,15,1865.
(12)Sohn,B.H.;Kim,K.;Choi,D.S.;Kim,Y.K.;Jeoung,S.C.; Jin,J.I.Macromolecules 2002,35,2876.
(13) Gruber,J.;Li,R.W.C.;Aguiar,L.H.J.M.C.;Benvenho,A.R. V.;Lessmann,R.;Huemmelgen,I.A.J.Mater.Chem.2005,15, 517.
(14)Shin,D.C.;Kim,Y.H.;You,H.;Kwon,S.K.Macromolecules 2003,36,3222.
(15) Chung,S.J.;Jin,J.I.;Lee,C.H.;Lee,C.E.Adv.Mater.1998, 10,684.
(16)Kwon,S.K.;Shin,D.C.;Kim,Y.H.;Kim,J.W.;Joo,D.J.; You,H.;Choi,D.S.Polym.Prepr.(Am.Chem.Soc.,Div. Polym.Chem.)2002,43,603.
(17) Johansson,D.M.;Srdanov,G.;Yu,G.;Theander,M.;Inganäs, O.;Andersson,M.R.Macromolecules 2000,33,2525.
(18) Johansson,D.M.;Theander,M.;Srdanov,G.;Yu,G.;Inganäs, O.;Andersson,M.R.Macromolecules 2001,34,3716.
(19) Lee,J.H.;Yu,H.S.;Kim,W.;Gal,Y.S.;Park,J.H.;Jin,S.H. J.Polym.Sci.,Part A:Polym Chem.2000,38,4185.
(20)Jin,S.H.;Jung,H.H.;Hwang,C.K.;Koo,D.S.;Shin,W.S.; Kim,Y.I.;Lee,J.W.;Gal,Y.S.J.Polym.Sci.A 2005,43,5062.
(21) Mo,Y.Q.;Huang,J.;Jiang,J.X.;Deng,X.Y.;Niu,Y.H.;Cao, Y.Chin.J.Polym.Sci.2002,20,461.
(22) Khanapure,S.P.;Biehl,E.R.J.Org.Chem.1987,52,1333.
(23)Wudl,F.;Khemani,K.C.;Harlev,E.;Ni,Z.;Srdanov,G. Polym.Mater.Sci.Eng.1991,64,201.
(24) Becker,H.;Spreitzer,H.;Ibrom,K.;Kreuder,W. Macromolecules 1999,32,4925.
(25) Fleissner,A.;Stegmaier,K.;Melzer,C.;von Seggern,H.; Schwalm,T.;Rehahn,M.Chem.Mater.2009,21,4288.
(26) Bredas,J.L.;Silbey,R.;Boudreaux,D.S.;Chance,R.R.J.Am. Chem.Soc.1983,105,6555.
November 25,2010;Revised:March 15,2011;Published on Web:April 15,2011.
Synthesis and Electroluminescent Properties of Phenyl-Substituted Poly(phenylenevinylene)
MO Yue-Qi1,*CHANG Xue-Yi2HU Su-Jun3HAN Shao-Hu3WU Hong-Bin3,*PENG Jun-Biao3
(1Key Laboratory of Special Functional Materials,South China University of Technology,Guangzhou 510640,P.R.China;2Canton Oledking Optoelectronic Materials Co.,Ltd.,Guangzhou 510730,P.R.China;3Institute of Polymer Optoelectronic Materials and Devices,South China University of Technology,Guangzhou 510640,P.R.China)
Poly(phenylenevinylene)(PPV)is one of the most efficient electroluminescent conjugated polymers and has the potential to be commercialized as important components in full-color flat panel displays.Here,we report an alternative synthetic route to the commercially available PPV derivative Super Yellow PPV(SY PPV)using 2-bromo-1,4-xylylene diacetate as a novel starting material.The intermediates, monomers,and the polymer were fully characterized by nuclear magnetic resonance(NMR)and elemental analysis.The SY PPV film shows an absorption maximum at 434 nm and an absorption onset at 510 nm with an optical bandgap of ca 2.44 eV.The photoluminescent and electroluminescent maximum of SY PPV are around 516 and 552 nm,respectively.The SY PPV obtained via this new synthetic route exhibits improved electroluminescent properties,a turn on voltage of over 2.4 V,a luminance maximum exceeding 49000 cd·m-2,and a maximum luminous efficiency(LE)of 21 cd·A-1compared with the SY PPV prepared by the traditional route(16-18 cd·A-1).
Polyphenylenevinylene;Phenyl-substituted polyphenylenevinylene;Green light emitting polymer;Polymer light-emitting device;Electroluminescence
O644
∗Corresponding authors.MO Yue-Qi,Email:moyueqi@263.net.WU Hong-Bin,Email:hbwu@scut.edu.cn;Tel:+86-20-87114400.
The project was supported by the National Natural Science Foundation of China(20674021)and the Specialized Research Fund for the Doctoral Program of Higher Education,China(20090172120012).
国家自然科学基金(20674021)和高等学校博士学科点专项科研基金新教师基金课题(20090172120012)资助项目
