碱土金属对ZrO2-Al2O3及其负载Pd-Rh密偶催化剂的影响
2011-11-30何胜楠崔亚娟姚艳玲方瑞梅史忠华龚茂初陈耀强
何胜楠 崔亚娟 姚艳玲 方瑞梅 史忠华 龚茂初 陈耀强
(四川大学化学学院,绿色化学与技术教育部重点实验室,成都610064)
碱土金属对ZrO2-Al2O3及其负载Pd-Rh密偶催化剂的影响
何胜楠 崔亚娟 姚艳玲 方瑞梅 史忠华*龚茂初 陈耀强*
(四川大学化学学院,绿色化学与技术教育部重点实验室,成都610064)
采用胶溶法制备了一系列碱土金属改性的ZrO2-Al2O3,并以其为载体采用等体积浸渍法制备了Pd-Rh密偶催化剂.采用低温N2吸附-脱附、X射线衍射(XRD)、氨气程序升温脱附(NH3-TPD)对载体样品进行了表征.结果表明,碱土金属的添加增大了ZrO2-Al2O3的比表面积,Sr-Zr-Al样品经1000°C焙烧5 h后具有最大的比表面积,为164 m2·g-1.对催化剂进行了H2程序升温还原(H2-TPR)、X射线光电子能谱(XPS)和活性表征,考察了催化剂对C3H8的转化活性.测试结果表明,添加碱土金属能有效提高催化剂上丙烷的转化活性.
碱土金属;ZrO2-Al2O3;Pd-Rh;密偶催化剂;丙烷
1 Introduction
Automobile exhaust gas is one of the major sources of atmosphere pollution.Since 1970s,the automobile exhaust catalysts have been widely used to purify exhaust gas.1,2Currently catalysts that meet more stringent emission standards generally consist of two parts:three-way catalysts(TWCs)and closecoupled catalysts(CCCs).The CCC is mainly to remove the hydrocarbons(HCs)during the cold start,because the majority (more than 80%)of the HCs is released during the cold start.3Moreover as the CCC is close to the engine,the catalyst support and active washcoat should be kept stable at high temperature when the engine works at high speed.
Researchers have tried many methods to improve catalytic activity,such as improving noble metal dispersion with high surface area supports,improving thermal stability of supports and so on.4As well known,Al2O3is widely used as catalyst support.Due to long time exposed to high temperature(sometimes even above 1000°C)exhaust gas and the high humidity atmosphere,Al2O3would gradually sinter and translate from γ-phase to α-phase and the surface area and thermal stability are all decreased.In order to stabilize Al2O3,some stabilizers need to be added.Researches showed that introducing alkali metals,alkaline earth metals,and rare earth elements can increase the thermal stability and surface area of alumina.5-9The alkaline earth metals which can stabilize Al2O3are Ba2+,Sr2+,and Ca2+.10Ba2+is the best.In addition,zirconia can stabilize alumina as well. Horiuchi et al.11have reported that the surface area of zirconia stabilized alumina,calcined at 1200°C,kept at 50 m2·g-1.However,using the alkaline earth metals and zirconia co-stabilized Al2O3as support for automotive exhaust catalyst in CCC has not been reported.
In this study,M-Zr-stabilized(M=Mg,Ca,Sr,Ba)Al2O3were prepared by peptizing method,and used as support to prepare Pd-Rh close-coupled catalysts.The influence of alkaline earth metals on textural properties of ZrO2-Al2O3was studied and catalytic activities of Pd-Rh close-coupled catalysts on C3H8conversion were investigated.
2 Experimental
2.1 Modified alumina preparation
A series of alumina stabilized by alkaline earth metals and zirconia(AR)were prepared by peptizing method,12using boehmite as precursor and nitric acid as peptizer.The mass fraction(w)of alkaline earth metal was 4%and that of zirconia was 6%.The products were dried at 105°C overnight and calcined at 600°C for 3 h in muffle furnace.In order to compare their thermal stabilities,the samples were calcined at 1000°C for 5 h.The products are referred as Zr-Al,Mg-Zr-Al,Ca-Zr-Al,Sr-Zr-Al,and Ba-Zr-Al.
2.2 Catalyst preparation
Catalysts were prepared by the impregnation method.13Different supports calcined at 1000°C were impregnated with the desired amounts of Pd(NO3)2and Rh(NO3)3aqueous solutions. And then some deionized water was added to the catalyst powders which were prepared above,the mixture was ground to form slurry.The resulting slurry was spread to a honeycomb cordierite(2.5 cm3,Corning,China),and then excessive slurry was blown away with compression air.Fresh catalysts were obtained by calcining at 550°C for 3 h in muffle furnace,and the aged catalysts were obtained by calcining at 1000°C for 5 h. The catalysts were denoted respectively as Pd-Rh/Zr-Al,Pd-Rh/ Mg-Zr-Al,Pd-Rh/Ca-Zr-Al,Pd-Rh/Sr-Zr-Al,and Pd-Rh/Ba-Zr-Al.The precious metal content was 2 g·L-1(nPd:nRh=19:1).
2.3 Characterization of supports and catalysts
2.3.1 Support characterization
The BET specific surface area and pore volume measurement were performed on a ZXF-5 automatic surface analyzer (Xibei Chemical Institute,China).The BET specific surface areas and pore volumes of samples were determined by using a N2adsorption technique at liquid nitrogen temperature.In order to desorb surface impurities,samples were degassed at 350°C for 1 h before the measurement.
The crystal structures of the samples were determined by a powder X-ray diffraction(XRD)(Rigaku International Corp., Japan)on a D/MaX-rA rotary diffractometer using Cu Kαradiation(λ=0.154 nm)and operated at 40 kV and 25 mA.The XRD data were tested for 2θ values between 10°and 90°with a rate of 0.03(°)·s-1.The crystalline phases were compared with the reference data from the International Center for Diffraction Data(ICDD).The particle size calculations were performed using peak-broadening analysis.
The NH3-TPD experiments were performed for supports. The sample(80 mg)was pretreated in a flow of Ar(30 mL· min-1)atatemperaturerangingfromroomtemperatureto400°C and was maintained at 400°C for 60 min.Then samples adsorbed NH3at the flow of 20 mL·min-1for 60 min after they were cooled to room temperature.Excessive NH3was blown off in Ar(30 mL·min-1).At last the temperature of the sample was increased to 500°C at the rate of 8°C·min-1,NH3was measured by a thermal conductivity detector(TCD).
2.3.2 Catalyst characterization
The CCC catalytic activity was evaluated in a multiple fixed-bed continuum flow micro-reactor by passing a gas mixture from a gasoline engine,which was similar to that of an exhaust from a gasoline engine.Before entering a blender,the gases were regulated using mass-flow controllers.The simulated exhaust contained a mixture of O2(adjustable),CO(0.86%), C3H8(0.06%),NO(0.12%),CO2(12%),H2O(10%),and N2(remaining).The gas space velocity was 34000 h-1.The concentration of C3H8was analyzed by a five-component analyzer (FGA-4100,Foshan)before and after the stimulated gas passed themicro-reactor.
The H2-TPR tests were performed for catalysts and detected by TCD-GC(gas chromatography).Each catalyst(100 mg) was put into a U-type quartz reaction tube and covered with quartz sand.After being preheated in N2atmosphere at 400°C for 60 min,it was cooled to room temperature,and then subjected to a flow of mixed gases of 5%H2-95%N2at the rate of 30 mL·min-1.The temperature of the sample was increased to 600°C at the rate of 8°C·min-1.
The X-ray photoelectron spectroscopy(XPS)experiments were carried out on a spectrometer(XSAM-800,KRATOS Co) with Mg Kαradiation under ultra-high vacuum condition.All samples were pretreated in a stream of 5%H2-95%N2at 400°C for 60 min,and cooled to room temperature.The binding energy was determined by reference to the C 1s binding energy of 284.8 eV.
3 Results and discussion
3.1 Textural properties of supports
The textural properties of alkaline earth modified zirconiaalumina after being calcined at 1000°C for 5 h are shown in
Table 1.It can be seen from Table 1 that the addition of alkaline earth metals increases the surface area of alumina samples. The surface area can even reach 164 m2·g-1when Sr is added. The surface area of Ba-Zr-Al is almost the same as that of Sr-Zr-Al,only the pore volume is a little smaller,0.45 cm3·g-1. It is to say that the addition of alkaline earth metal plays an important role in stabilizing alumina,especially Sr and Ba.The ionic radii of the alkaline earth metals increase in turn from Mg to Ba(0.065,0.099,0.113,and 0.135 nm,respectively).In a certain amount range,the introduction of doped ions in alumina can prevent the migration of O2-ions and Al3+ions thus stabilize alumina at high temperature.And the larger the ionic radius is,the better the stability effect is.So the surface areas of the samples from Mg to Ba increase in turn.However,introducing alkaline earth metals into Zr-Al has little influence on their pore volumes.
3.2 XRD analysis
The powder XRD patterns of the alumina samples are shown in Fig.1.The 2θ angles of 37.5°,45.5°,and 67.2°display the diffraction peaks of γ-Al2O3.The 2θ angles of 25.5°, 43.4°,and 57.5°show the diffraction peaks of α-Al2O3.And the diffraction peaks of ZrO2appear in the 2θ angles of 30.2°, 35.2°,50.3°,60.2°,and 81.8°.Zr-Al samples mainly displayγ-Al2O3and small amount of α-Al2O3and ZrO2.It demonstrates that the Al2O3and ZrO2did not fully form to solid solution,but separate into two phases.ZrO2plays a role in stabilizing Al2O3, which is mainly due to the fact that ZrO2grains disperse in the surface of Al2O3grains,and they prevent particle agglomeration rather than forming solid solution to stabilize the alumina.11After the introduction of alkaline earth metals,none of the diffraction peaks is observed in the XRD patterns of all samples.And this is mainly owe to two reasons,one is that alkaline earth metal oxide grains are too small and scatter better, and can not show a diffraction peak.The other is that the alkaline earth metal ions enter into the alumina grains or the zirconia grains to form alumina or zirconia solid solution.No matter what form they consist,these two factors can all stabilize alumina.They can prevent particle agglomeration sintering and the migration of Al3+ions.So the alumina with a high surface area and pore volume can be maintained at high temperature with the addition of them.It is consistent with the BET results.
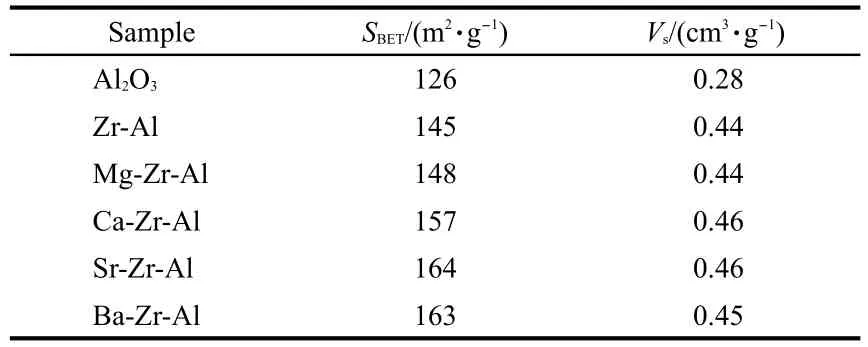
Table 1 Textural properties of alkaline earth metal modified zirconia-alumina after calcination at 1000°C for 5 h
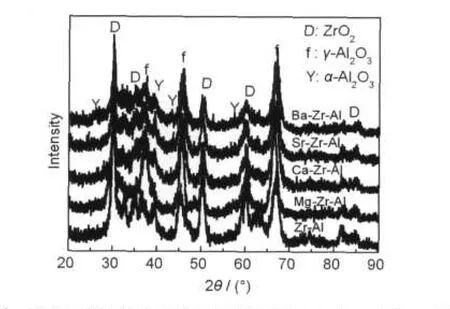
Fig.1 X-ray diffraction patterns of alkaline earth metal modified zirconia-alumina after calcination at 1000°C for 5 h
Fig.2 shows the X-ray diffraction patterns of alkaline earth metal modified zirconia-alumina after calcination at 1000°C for 5 h.As shown in Fig.2(a),the peak at 2θ angle of 30.2°is the diffraction peak of zirconia(111).The diffraction peak intensity decreases and peak width increases after the introduction of the alkaline earth metal,but there is no obvious shift.Results indicate that ZrO2grains become smaller with the addition of alkaline earth metals,and the alkaline earth metal ions do not enter into the lattice of zirconia.
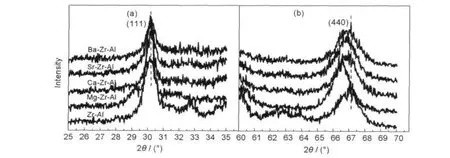
Fig.2 Powder X-ray diffraction patterns of alkaline earth metal modified zirconia-alumina after calcination at 1000°C for 5 h 2θ:(a)25°-35°,(b)60°-70°
As seen from Fig.2(b),the peak at the 2θ angle of 67.2°is the diffraction peak of γ-Al2O3(440).And the peak shows an apparent shift to small angle when the alkaline earth metal is introduced.Especially,the γ-Al2O3diffraction peak of Mg-Zr-Al offset relatively larger,the 2θ angle deviates nearly 0.5°;besides,the other three samples also have a different degree of deviation.It indicates that the alkaline earth metal ions have entered the lattice of alumina and changed lattice parameters.The ion radii of alkaline earth metals are larger than that of aluminum,as a result,the lattice of alumina will be expand,and the peak moves forward to smaller diffraction angle.The ion radius of Mg and Al is similar to each other.It is easy to form solid solution.So the Mg-Zr-Al has the maximum diffraction angle deviation.
3.3 NH3-TPD analysis
Fig.3 shows the NH3-TPD of the alumina samples caclined at 1000°C for 5 h.The desorption peak of the Zr-Al samples not only has the most wide temperature range from 130 to 450°C,but also has the largest peak area.When alkaline earth metal is introduced,both the peak temperature and the peak area all decrease from Mg to Ba,and their highest peak temperatures are 180,175,149,and 147°C,respectively. Looking at the peak from another view,the desorption peak is asymmetric.It indicates that the desorption peak is formed by two overlapping peaks,while both the Sr-Zr-Al and Ba-Zr-Al show two separate peaks.All of these indicate that the surface of alumina samples have two acidic sites.One is a weak acid place;another is a middle strong acid site.
Alkaline earth metal oxides are basic compounds,and the alkaline increases gradually with the atomic number increasing.In addition,the surface acid sites of alumina are mainly determined by the number of hydroxyl groups on the surface of aluminum and the aluminum atoms,the more hydroxyl groups and aluminum atoms,the more H+and empty electron orbital. Besides,the surface acidity of alumina will be enhanced with the acidic oxide ZrO2accession.The addition of alkaline earth metal oxides will first neutralize some strong and middle strong acid sites on the surface,and then neutralize the weak acid sites,resulting in a decline in the surface acidity.What is more,with the atomic number of alkaline additive enhancing the middle strong acid sites of alumina decrease,and the weak acid peak shifts to low temperature range.
3.4 Catalytic performance
The close-coupled catalytic performances for the C3H8oxidation are shown in Fig.4 and Fig.5.The catalytic properties of the C3H8conversion temperature are presented in Table 2.The order of the fresh catalytic activity is Pd-Rh/Ba-Zr-Al> Pd-Rh/Sr-Zr-Al>Pd-Rh/Ca-Zr-Al>Pd-Rh/Mg-Zr-Al>Pd-Rh/Zr-Al.It indicates that the catalytic activity will increase with the addition of alkaline earth metal.Obviously,the complete conversion temperature(T90)of catalysts with alkaline earth element is nearly 20°C lower than that of the Pd-Rh/Zr-Al catalyst.And the corresponding light-off temperature(T50)and T90of the Pd-Rh/M-Zr-Al catalysts were around 275 and 313°C, respectively;the ΔT(ΔT=T90-T50)is nearly 35°C.The activity increases rapidly with the temperature increasing and it can quickly reach conversion of the C3H8.That is,the Pd-Rh/MZr-Al catalysts have excellent temperature characteristics.It can also conclude from Fig.5 that the Pd-Rh/M-Zr-Al catalysts have better activity than the Pd-Rh/Zr-Al catalyst after they are all aged.Unfortunately,the temperature characteristics of aged catalysts declined a little with respect to the fresh catalysts. The catalysts were prepared with the same conditions except for the use of different supports.It is obviously that the supports led to the difference in activity.
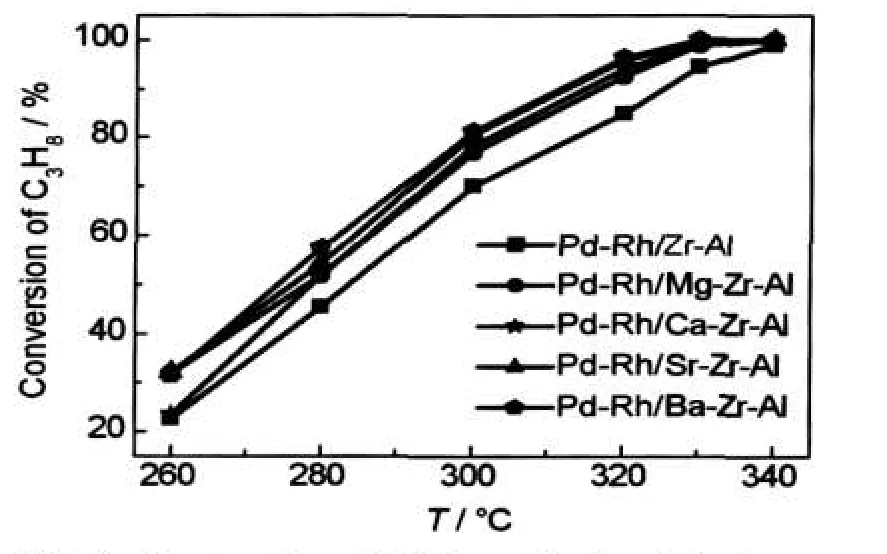
Fig.4 Conversion of C3H8on fresh catalysts
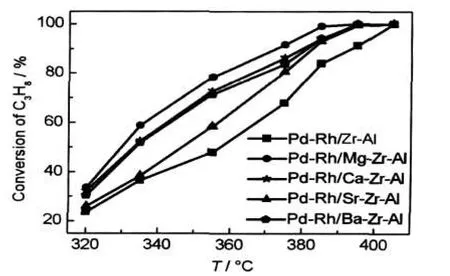
Fig.5 Conversion of C3H8on aged catalysts
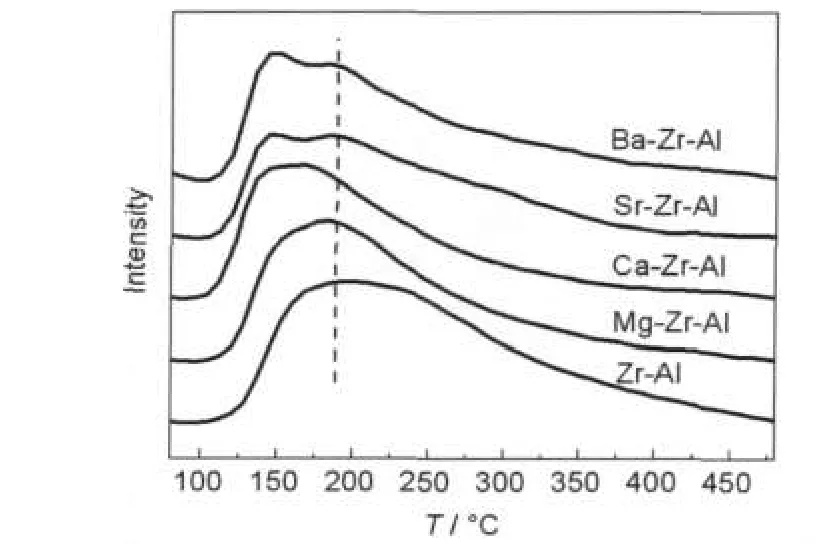
Fig.3 NH3-temperature programmed desorption profiles of alkaline earth metal modified zirconia-alumina after calcination at 1000°C for 5 h
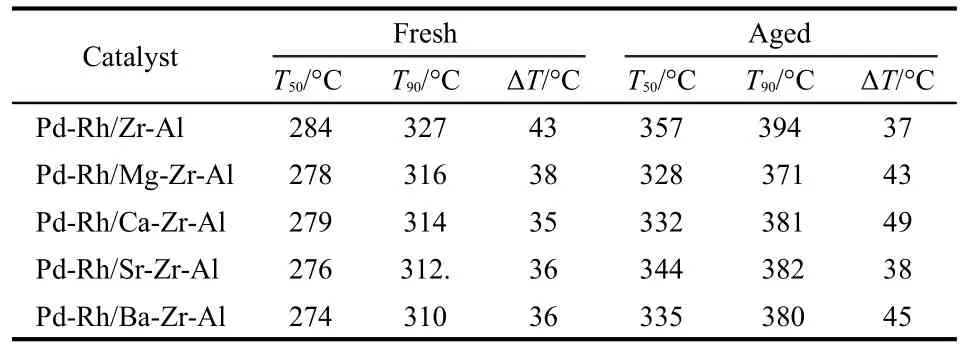
Table 2 T50and T90for the conversion of C3H8on different catalysts
The catalysts with the addition of alkaline earth metal have higher activity and thermal stability than the Pd-Rh/Zr-Al catalyst.The reasons are as follows:(1)the textural property plays an important role in the performance of catalyst,and the large surface area and pore volume are in favor for the better dispersion of noble metal,in addition,they are also in favor for mass and heat transfer.According to the textural property results,the Zr-Al with the alkaline earth metal has larger surface area,pore volume and better thermal stability than pure Zr-Al support after calcination at 1000°C.(2)The alkaline earth metal changes the support surface acid centers,resulting in a decline in the surface acidity and an increase in the basicity,thus, affecting the distribution of Pd,Rh active component.And the TPR results in the following text show that the introduction of alkaline earth metal increases the distribution of Pd,Rh,so that the number of catalyst active sites and catalytic activity increase.
Although the support alumina is calcined at the high temperature of 1000°C,the catalytic activity declined a little after being aged at 1000°C.Aging progress leaded to accumulating and sintering of precious metal.The introduction of Rh is easy to generate RhAlO3.14,15These results would give rise to the decline of the dispersion of precious metals.Therefore,the effective area of active component contacting with reactant molecules is reduced.As a result,the activity declines.
3.5 H2-TPR analysis
The reducibility of support Pd-Rh catalyst plays an important role in its catalytic property.It is generally acknowledged that the reduction temperature of precious metals is lower and the reduction peak area is larger(peak area corresponding to the adsorbed hydrogen consumption;and when the peak area is larger,the adsorbed hydrogen is more),thus the catalysts have better activity for catalytic reaction.13
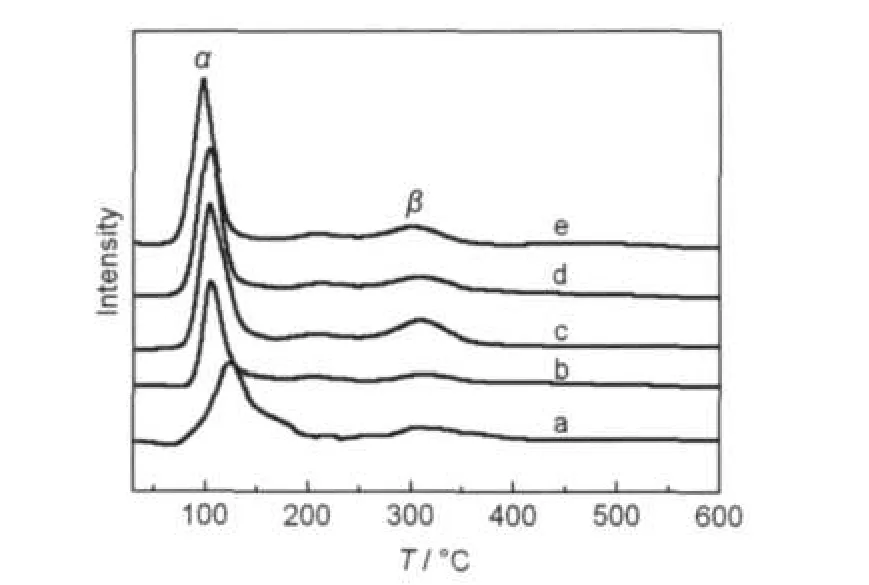
Fig.6 TPR profiles of the catalysts calcined at 550°C(a)Pd-Rh/Zr-Al,(b)Pd-Rh/Mg-Zr-Al,(c)Pd-Rh/Ca-Zr-Al,(d)Pd-Rh/Sr-Zr-Al, (e)Pd-Rh/Ba-Zr-Al
For α peak of catalysts,it is commonly attributed to reduction of PdO due to the interaction of Pd and ZrO2.16,17It can be seen from Fig.6 that the addition of alkaline earth metals makes the α peak shift to lower temperature,indicating that the addition of alkaline earths increases the reducibility of PdO species,and the peak area is larger than that in Pd-Rh/Zr-Al catalyst,that is,the amount of active component distribution in Pd-Rh/M-Zr-Al catalyst is larger than that of Pd-Rh/Zr-Al catalyst.So the Pd-Rh/M-Zr-Al catalyst has better catalytic activity than Pd-Rh/Zr-Al catalyst.In addition,the peak temperature decreases and peak area increases all from Mg to Ba in turn, that is,the order of Pd-Rh/M-Zr-Al catalytic activity is Pd-Rh/ Ba-Zr-Al>Pd-Rh/Sr-Zr-Al>Pd-Rh/Ca-Zr-Al>Pd-Rh/Mg-Zr-Al. For the β peak,the reduction temperature of Rh of all the catalysts is almost at the same temperature of 301°C.But the peak area increases with the addition of alkaline earths.
On the basis of the above results,the reduction temperature and peak area of the precious species in the catalysts decreases in the following order:Pd-Rh/Ba-Zr-Al>Pd-Rh/Sr-Zr-Al>Pd-Rh/Ca-Zr-Al>Pd-Rh/Mg-Zr-Al>Pd-Rh/Zr-Al.This order is also in accordance with that of their catalytic activity.
3.6 XPS analysis
In order to investigate the changes in the chemical states of Pd ions and Rh ions,the fresh catalysts were studied by XPS.
Fig.7 presents the Pd 3d photoelectron peaks of the catalysts. No Rh peaks are detected on the catalyst surface due to its low content.
We got the values of Pd 3d5/2level in an attempt to identify the chemical state of Pd.By comparing to the reference18where the Pd 3d binding energies have been measured both for metallic Pd,PdO,PdO2,and PdxO(x>1),the binding energy is about 336.2 eV for the impregnation catalyst.19It can be seen from Fig.7 that,on one hand,the Pd has different binding energies in different catalysts.That is,Pd has different oxidation states.The Pd 3d5/2has the highest oxidation state in Pd-Rh/ Zr-Al catalyst among all the catalysts,its Pd 3d5/2is 336.60 eV which is assigned to PdO(336.6 eV).20The Pd 3d5/2of the remaining four catalysts are 336.39,336.40,336.35,and 336.30 eV,which are between Pd0(335.0 eV)21and PdO(336.6 eV) and are more near to PdO.It indicates that the Pd is at lower oxidation states in those catalysts which include alkaline earth metal.As we all know,the lower the Pd binding energy,the higher the catalytic properties,22so the activity of the Pd-Rh catalyst with alkaline earth metal is higher than that of Pd-Rh/ Zr-Al catalyst.This is also consistent with catalytic activity test results.On the other hand,there is a decrease in the Pd 3d intensity of Pd-Rh/Zr-Al catalyst in comparison to the other four catalysts.It may be accounted for the possible PdO diffusion to the washcoat,sintering of Pd particles,and the structural changes inAl2O3support.
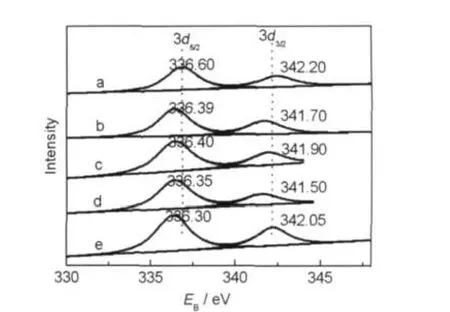
Fig.7 XPS profiles of Pd 3d of the catalysts calcined at 550°C (a)Pd-Rh/Zr-Al,(b)Pd-Rh/Mg-Zr-Al,(c)Pd-Rh/Ca-Zr-Al,(d)Pd-Rh/Sr-Zr-Al, (e)Pd-Rh/Ba-Zr-Al
4 Conclusions
Addition of alkaline earth metal plays an important role in stabilizing ZrO2-Al2O3.The surface area of support containing Sr or Ba reaches 160 m2·g-1after being calcined at 1000°C. XRD results indicate that the alkaline earth metal ions have entered into the lattice of alumina.They prevent the migration and diffusion of O2-ions and Al3+ions,so the γ phase of alumina is maintained.In addition,the Pd-Rh close-coupled catalysts doping with alkaline earth metal have better catalytic activity than the catalyst prepared with ZrO2-Al2O3as a support.
(1) Heck,R.M.;Farrauto,R.J.Appl.Catal.2001,221,443.
(2) Kašpar,J.;Fornasiero,P.;Hickey,N.Catal.Today 2003,77, 419.
(3) Farrauto,R.J.;Heck,R.M.Catal.Today 2000,55,179.
(4) Shinjoh,H.;Hatanaka,M.;Nagai,Y.;Tanabe,T.;Takahashi,N.; Yoshida,T.;Miyake,Y.Top.Catal.2009,52,1967.
(5) Wilcox,L.;Burnside,G.;Kiranga,B.;Shekhawat,R.; Mazumder,M.K.;Hawk,R.M.;Lindquist,D.A.Chem.Mater. 2003,15,51.
(6)Wu,X.D.;Yang,B.;Weng,D.J.Alloy.Compd.2004,376,241.
(7)Yue,B.H.;Zhou,R.X.;Zheng,X.M.Chin.J.Inorg.Chem. 2007,23,533.[岳宝华,周仁贤,郑小明.无机化学学报, 2007,23,533.]
(8) Liotta,L.F.;Macaluso,A.;Arena,G.E.;Livi,M.;Centi,G.; Deganello,G.Catal.Today 2002,75,439.
(9) Gong,M.C.;Wen,M.;Zhang,J.;Lin,Z.E.;Yang,Y.H.;Chen, Y.Q.Chin.J.Inorg.Chem.2001,17,50.[龚茂初,文 梅,章 洁,林之恩,羊彦衡,陈耀强.无机化学学报,2001,17, 50.]
(10) Church,J.S.;Cant,N.W.;Trimm,D.L.Appl.Catal.1993,101, 105.
(11) Horiuchi,T.;Teshima,Y.;Osaki,T.;Sugiyama,T.;Suzuki,K.; Mori,T.Catal.Lett.1999,62,107.
(12) Zhang,L.J.;Dong,W.P.;Guo,J.X.;Yuan,S.H.;Zhang,L.; Gong,M.C.;Chen,Y.Q.Acta Phys.-Chim.Sin.2007,23, 1738.[张丽娟,董文萍,郭家秀,袁书华,张 磊,龚茂初,陈耀强.物理化学学报,2007,23,1738.]
(13)Guo,J.X.;Gong,M.C.;Yuan,S.H.;Chen,Y.Q.J.Rare Earths 2006,24,554.[郭家秀,龚茂初,袁书华,陈耀强.稀土学报,2006,24,554.]
(14) Lassi,U.;Polvinen,R.;Suhonen,S.;Kallinen,K.;Savimäki,A.; Härkönen,M.;Valden,M.;Keiski,R.L.Appl.Catal.2004,263, 241.
(15) Zimowska,M.;Wagner,J.B.;Dziedzic,J.;Camra,J.; Borzecka-Prokop,B.;Najbar,M.Chem.Phys.Lett.2006,417, 137.
(16)Asakura,K.;Iwasawa,Y.J.Phys.Chem.1992,96,73.
(17) Narui,K.;Furuta,K.;Yata,H.;Nishida,A.;Kohtoku,Y.; Matsuzaki,T.Catal.Today 1998,45,173.
(18) Qin,D.;Lapszewicz,J.;Jiang,X.J.Catal.1996,159,140.
(19)Huang,W.;Zuo,Z.J.;Han,P.D.;Li,Z.H.;Zhao,T.D. J.Electr.Spectrosco.Relat.Phenom.2009,173,88.
(20) Benkhaled,M.;Morin,S.;Pichon,C.;Thomazeau,C.;Verdon, C.;Uzio,D.Appl.Catal.2006,312,1.
(21) Fujimoto,K.I.;Ribeiro,F.H.;Avalos-Borja,M.;Iglesia,E. J.Catal.1998,179,431.
(22) Li,X.;Meng,M.;Liu,Y.;Luo,J.Y.Chin.J.Catal.2007,28, 835.[李 想,孟 明,刘 咏,罗金勇.催化学报,2007,28, 835.]
January 10,2011;Revised:March 10,2011;Published on Web:March 30,2011.
Effects of Alkaline Earth Metal on Performance of ZrO2-Al2O3Support and Pd-Rh Close-Coupled Catalyst
HE Sheng-Nan CUI Ya-Juan YAO Yan-Ling FANG Rui-Mei SHI Zhong-Hua*GONG Mao-Chu CHEN Yao-Qiang*
(Key Laboratory of Green Chemistry&Technology of the Ministry of Education,College of Chemistry,Sichuan University, Chengdu 610064,P.R.China)
A series of alumina supports stabilized by alkaline earth metals and zirconia were prepared by the peptizing method.Pd-Rh close-coupled catalysts supported on modified alumina were prepared by the impregnation method.The supports were characterized by low temperature nitrogen adsorption-desorption method,X-ray diffraction(XRD),and NH3-temperature programmed desorption(NH3-TPD).For the catalysts,H2-temperature programmed reduction(H2-TPR)and X-ray photoelectron spectroscopy(XPS) were carried out.We also carried out catalytic activity tests for C3H8conversion.Results show that the addition of alkaline earth metals increases the surface area of the supports and the surface area of Sr-Zr-Al reaches 164 m2·g-1after calcination at 1000°C for 5 h.The introduction of alkaline earth metals into the ZrO2-Al2O3supports also improves their catalytic activity toward propane oxidation and the activities of the Pd-Rh catalysts containing alkaline earth metals are higher than that of the catalyst prepared with a ZrO2-Al2O3support.
Alkaline earth metal;ZrO2-Al2O3;Pd-Rh;Close-coupled catalyst;Propane
O643
*Corresponding authors.CHEN Yao-Qiang,Email:nic7501@scu.edu.cn;Tel/Fax:+86-28-85418451.SHI Zhong-Hua,Email:shizh96@scu.edu.cn. The project was supported by the National Natural Science Foundation of China(20773090,20803049)and Specialized Research Fund for the
Doctoral Program of Higher Education,China(20070610026).
国家自然科学基金(20773090,20803049)和教育部博士点新教师基金(20070610026)资助项目
