Hydrothermal Syntheses and Crystal Structures of Two Metal Complexes with Mixed 1,2,4-Benzenetricarboxylic Acid and 1,3,5-Tris(1-imidazolyl)benzene Ligands
2011-11-09SUZhiLGaoChaoOKAMURATakiakiCHENManShengCHENShuiShengSUNWeiYin
SU ZhiLÜ Gao-ChaoOKAMURA Taki-akiCHEN Man-ShengCHEN Shui-ShengSUN Wei-Yin*,
(1Coordination Chemistry Institute,State Key Laboratory of Coordination Chemistry,School of Chemistry and Chemical Engineering,Nanjing University,Nanjing 210093,China) (2Department of Macromolecular Science,Graduate School of Science,Osaka University,Toyonaka,Osaka 560-0043,Japan)
Hydrothermal Syntheses and Crystal Structures of Two Metal Complexes with Mixed 1,2,4-Benzenetricarboxylic Acid and 1,3,5-Tris(1-imidazolyl)benzene Ligands
SU Zhi1LÜ Gao-Chao1OKAMURA Taki-aki2CHEN Man-Sheng1CHEN Shui-Sheng1SUN Wei-Yin*,1
(1Coordination Chemistry Institute,State Key Laboratory of Coordination Chemistry,School of Chemistry and Chemical Engineering,Nanjing University,Nanjing210093,China) (2Department of Macromolecular Science,Graduate School of Science,Osaka University,Toyonaka,Osaka560-0043,Japan)
Two new coordination complexes[Co(1,2,4-HBTC)(tib)](1)and[Ni4(1,2,4-BTC)2(tib)4(H2O)2]·(1,2,4-HBTC)·9H2O(2)(1,2,4-H3BTC=1,2,4-benzenetricarboxylic acid,tib=1,3,5-tris(1-imidazolyl)benzene)were obtained by hydrothermal reactions of 1,2,4-H3BTC and tib with corresponding metal salts.Single crystal X-ray diffraction analysis revealed that each tib ligands connect three metal atoms to form two-dimensional(2D)networks in 1 and 2.On the other hand,each 1,2,4-HBTC2-coordinates to one Co(Ⅱ)atom as a terminal ligand in 1,while in 2,two 1,2,4-BTC3-ligands link four Ni(Ⅱ)atoms which further connect[Ni(tib)]2+2D networks to result in formation of multi-layered 2D structure.Complexes 1 and 2 are finally extended to three-dimensional(3D)supramolecular structures by hydrogen bonding interactions.CCDC:816549,1;815931,2.
1,2,4-benzenetricarboxylic acid;1,3,5-tris(1-imidazolyl)benzene acid;crystal structure;hydrothermal synthesis
In recent years,the design and synthesis of metalorganic frameworks (MOFs)constructed from metal salts and bridging organic ligands have attracted great attention,not only because of their intriguing variety of architectures and topologies[1-5],but also because of their potential applications in ion-exchange,nonlinear optics, gas storage,catalysis,magnetism and molecular sensing[6-10].Consequently,a variety of MOFs with inter-esting structures and topologies have been prepared by taking certain factors into account,for example the coordination nature of the metal ion and the shape, functionality,flexibility of organic ligand and so on[11-12]. From the viewpoint of crystal engineering,the most effective approach may be the appropriate choice of well-designed organic ligands containing modifiable backbones and connectivity,together with the metal centers with definite coordination preferences[13-15].On the other hand,mixed ligands with different donor groups have been proved to be effective building units to construct novel coordination polymers,because they have strong coordination affinity and can satisfy the geometric need of metal centers[16-19].According to the above consideration and to further investigate the influence of organic ligands on thecoordination architectures,reactions of varied metal salts with 1,2,4-benzenetricarboxylic acid and 1,3,5-tris(1-imidazolyl) benzene mixed ligands were carried out.Herein we report the synthesis,crystal structure and thermogravimetric analysis of two coordination polymers, [Co(1,2,4-HBTC)(tib)](1)and[Ni4(1,2,4-BTC)2(tib)4(H2O)2]·(1,2,4-HBTC)·9H2O(2)(1,2,4-H3BTC=1,2,4-benzenetricarboxylic acid,tib=1,3,5-tris(1-imidazolyl) benzene).
1 Experimental
1.1 Materials and instruments
The regents and solvents were used as commercial sources without further purification.The tib ligand was prepared according to the method reported previously[20]. Elemental analyses were performed on a Perkin-Elmer 240C elemental analyzer.The IR spectra were recorded on Bruker Vector22 FTIR spectrophotometer using KBr discs.Thermogravimetric analyses (TGA)were performed on a TGA V5.1A Dupont 2100 instrument heating from room temperature to 700℃under N2with a heating rate of 20℃·min-1.
1.2 Syntheses of the compounds 1 and 2
Complex 1 was synthesized by hydrothermal method in a 16 mL Teflon-lined autoclave by heating a mixture containing Co(ClO4)2·6H2O(73.1 mg,0.2 mmol),1,2,4-H3BTC(21.2 mg,0.1 mmol),tib(27.3 mg, 0.1 mmol)and NaOH (12.0 mg,0.3 mmol)in 10 mL H2O at 180℃for 3 d.Violet block single crystals of 1 were collected with a yield of 42%by filtration and washed by water and ethanol for several times.Anal. Calcd.for C24H16CoN6O6(%):C,53.00;H,2.94;N, 15.46.Found(%):C,53.08;H,2.89;N,15.49%.IR (KBr pellet,cm-1):3 410(s,br),1 721(s),1 621(s),1 598 (s),1 513(m),1 377(m),1 324(w),1 256(m),1 233(m), 1 105(s),1 016(m),950(m),744(m),684(w),651(m), 622(m).
The preparation of 2 is similar to that of 1 except that Ni(ClO4)2·6H2O(75.1 mg,0.2 mmol),instead of Co(ClO4)2·6H2O,was used.Blue block crystals of 2 were obtained in 34%yield after washing by water and ethanol for several times.Anal.Calcd.for C87H80N24Ni4O29(%):C,48.32;H,3.70;N,15.55.Found(%):C, 48.12;H 3.64;N,15.69.IR(KBr pellet,cm-1):3410(s, br),1720(s),1618(s),1572(s),1515(s),1 394(s),1 318 (m),1243(s),1 110(m),1 074(s),1 018(m),950(m),854 (m),763(m),749(m),650(m).
1.3 Structure determination
The crystallographic data collections for complexes 1 and 2 were carried out on a Rigaku RAXIS-RAPID Imaging Plate diffractometer with graphitemonochromated Mo Kα radiation(λ=0.071075 nm)at 200 K using the ω-scan technique.The structures were solved by direct methods using SIR92 and expanded using Fourier techniques[21-22].All non-hydrogen atoms were refined anisotropically.All calculations were performed using the Crystal Structure (Version 3.8) crystallographic software package except for the refinement,which was performed using SHELXL-97[23-24]. Hydrogen atoms of the ligands tib and 1,2,4-BTC3-in the structureswere generated geometrically.The hydrogen atom of non-deprotonated 1,2,4-HBTC2-was located directly in 1(H1),and was not found in 2.The hydrogen atoms of water molecules in 2 were also not found except for O11 and O12 with attached hydrogen atoms found in the differential Fourier map and located. Each atom of C311,C322,O7,O8,O9 and O10 in 2 has the site occupancy factors of 0.75.And atom O15 has site occupancy of 0.5 and O16 in 2 was disordered into two positions with the site occupancy factors of 0.60(3)and 0.40(3),respectively.Crystal data and structure refinement parameters are listed in Table 1.The selected bond lengths and bond angles are given in Table 2.
CCDC:816549,1;815931,2.

Table 1 Crystal data and structure parameters for complexes 1 and 2
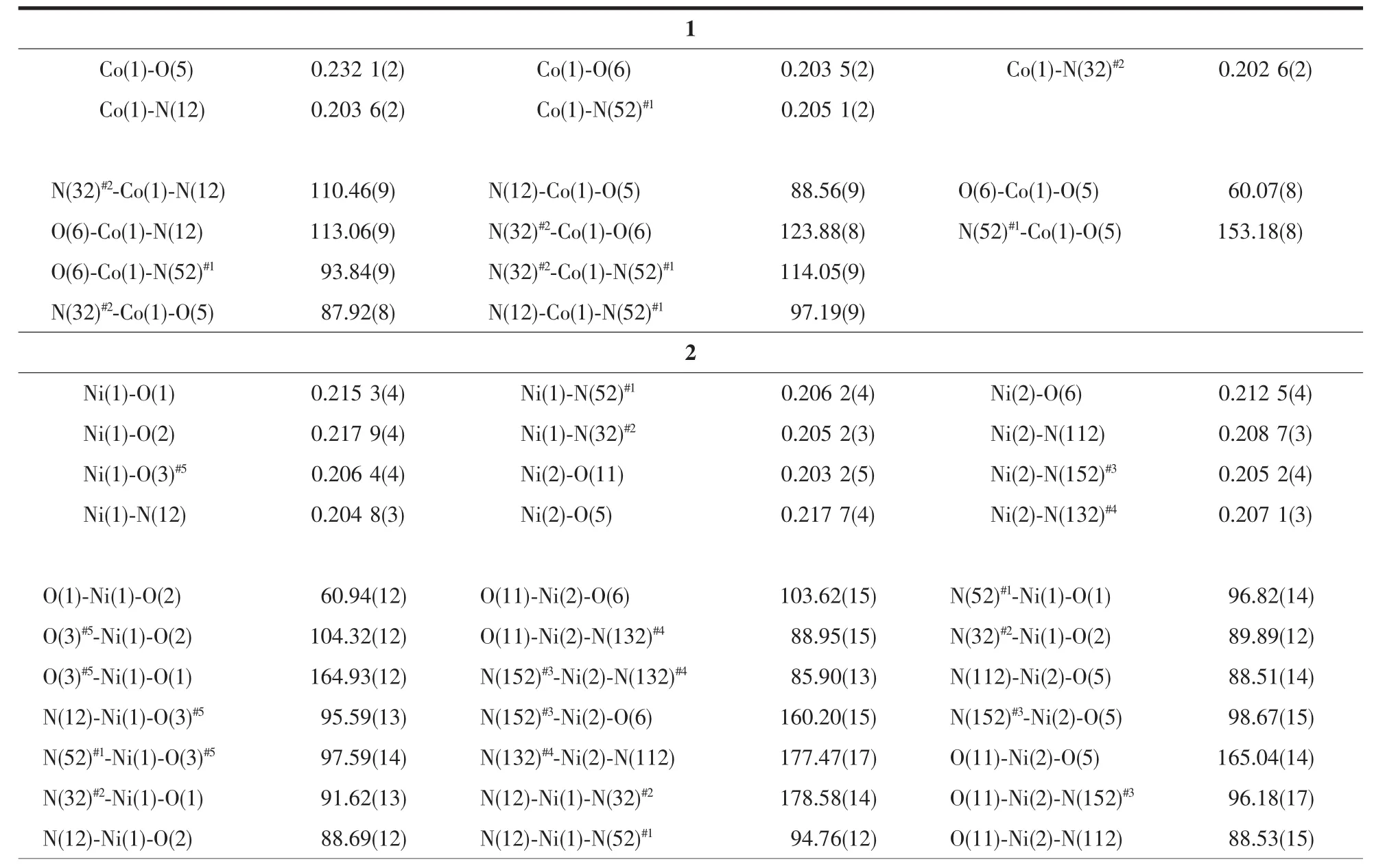
Table 2 Selected bond lengths(nm)and bond angles(°)
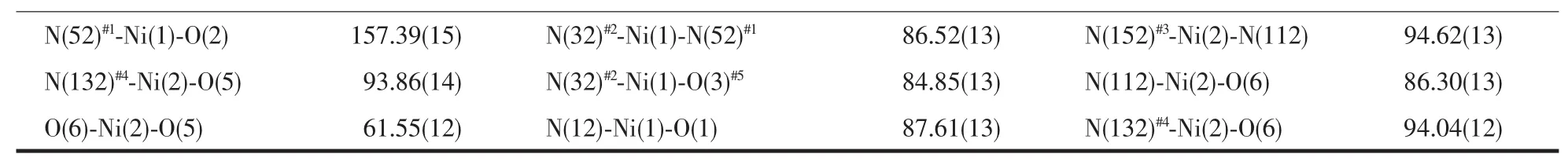
Continued Table 2
2 Results and discussion
2.1 Crystal structure description
The results of structural analysis revealed that complex 1 has a two-dimensional(2D)layer structure. The asymmetric unit of 1 contains one Co(II)atom,one tib and one partially deprotonated 1,2,4-HBTC2-ligand. The presence of non-deprotonated carboxylic group (-COOH)of 1,2,4-HBTC2-in 1 was also confirmed by IR spectral data (vide post).As shown in Fig.1,the central Co(Ⅱ)atom has distorted square-pyramidal coordination environment as indicated by an index τ value of 0.49[25]and is coordinated by two oxygen and two nitrogen atoms(O5,O6,N52#1and N32#2)in the equatorial plane and the other nitrogen atom (N12)in the axial position.Two Co-O bond distances are 0.2321(2), 0.2035(2)nm and three Co-N ones are 0.2026(2), 0.203 6(2)and 0.205 1(2)nm,respectively(Table 2). On the other hand,each tib ligand acts as a threeconnector to link three Co(Ⅱ) atoms to form a 2D network with(4,82)topology(Fig.2).It is noteworthy that each 1,2,4-HBTC2-uses only one carboxylate group to coordinate with one Co(Ⅱ) atom as a terminal ligand. Thus the final structure of 1 is a 2D network as exhibited in Fig.2.
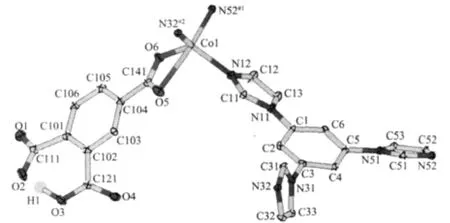
Fig.1 Coordination environment of Coin 1 with ellipsoids drawn at the 30%probability level
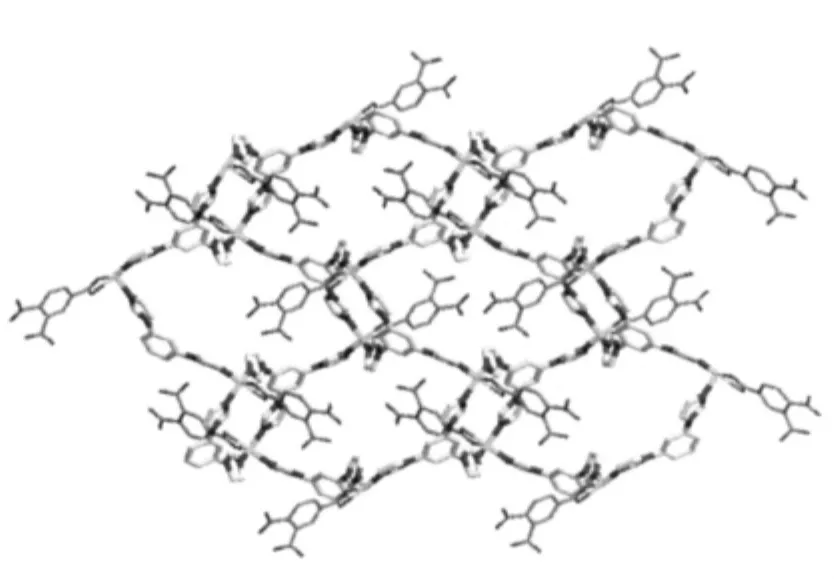
Fig.2 2D network of 1 with all hydrogen atoms omitted for clarity

Fig.3 Coordination environment of Niin 2 with ellipsoids drawn at the 30%probability level
When Ni(ClO4)2·6H2O wasused,instead of Co(ClO4)2·6H2O,to react with ligand tib and 1,2,4-H3BTC,complex 2 with different 2D structure was obtained.The asymmetric unit of 2 contains two Ni(Ⅱ)atoms,two tib,one coordinated 1,2,4-BTC3-and half non-coordinated 1,2,4-HBTC2-,one coordinated and four and half free water molecules.The coordination environment of Ni(Ⅱ) in 2 is shown in Fig.3.It can be seen that both Ni(1)and Ni(2)have similar distorted octahedral coordination environments, which is obviously different from the distorted square-pyramidal Co(Ⅱ)in 1.Each Ni(1)atom is coordinated by three nitrogen (N12,N52#1and N32#2)atoms from three different tib ligands and three carboxylate oxygen(O1, O2 and O3#5)atoms from two distinct 1,2,4-BTC3-ligands,while Ni(2)is coordinated by three nitrogen (N112,N152#3and N132#4)atoms from three different tib ligands and two carboxylate oxygen (O5,O6)atoms from one 1,2,4-BTC3-and one oxygen atom(O11)from a terminal water molecule with Ni-N bond lengths ranging from 0.2048(3)to 0.2087(3)nm,the Ni-O ones in the range of 0.203 2(5)~0.217 9(4)nm and bond angles around the Ni(Ⅱ)in the range of 60.94(12)°to 178.58(14)°(Table 2).It is interesting to find that each tib ligand coordinates to three Ni(Ⅱ) atoms,however, leading to the formation of typical 63-hcb 2D network (Fig.4),rather than the(4,82)one in 1.Furthermore,the coordination mode of 1,2,4-BTC3-ligand in 2 is completely different from that in 1.Each 1,2,4-BTC3-in 2 connects three Ni(Ⅱ) atoms using its three carboxylate groups,and two 1,2,4-BTC3-ligands and four Ni(Ⅱ)atoms form a Ni4(1,2,4-BTC)2subunit which further links adjacent four Ni-tib 2D networks to result in formation of a multi-layered 2D structure of 2(Fig.4).The non-coordinated 1,2,4-HBTC2-and water molecules are located between the two adjacent 2D layers.
Both 2D networks of1 and 2 arefurther connected together through hydrogen bonding interactions(Table 3)to give the corresponding threedimensional (3D)supramolecularstructures.The different structures of complexes 1 and 2 imply that the metal centers play crucial role in the formation of the frameworks 1 and 2.

Fig.4 2D structure of 2
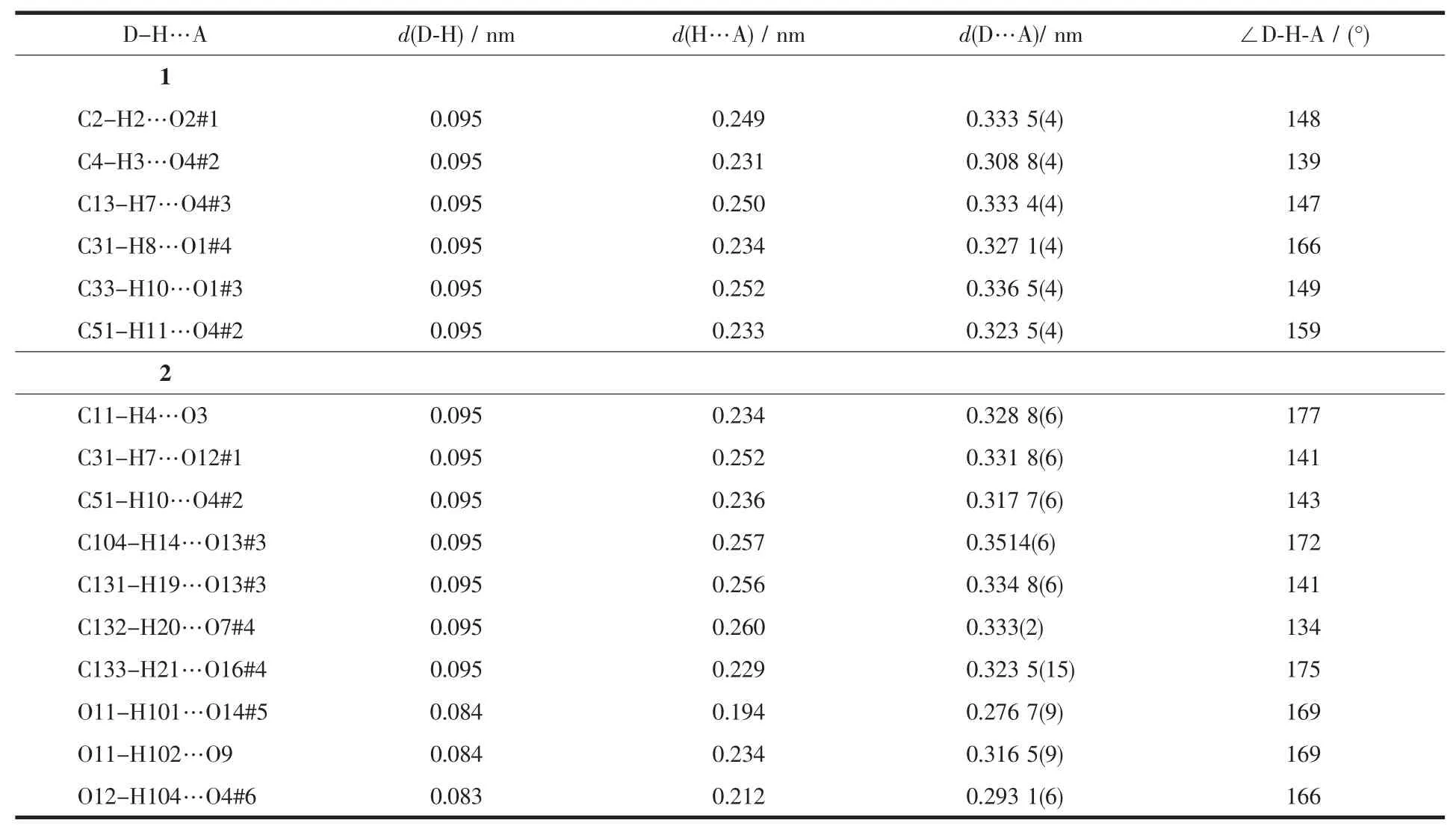
Table 3 Hydrogen bonding data for complexes 1 and 2
2.2 IR and thermogravimetric analyses
The infrared spectra(IR)of the complexes 1 and 2 have been recorded and strong IR bands from-COOH were observed at 1 721 and 1 720 cm-1,respectively, indicating the non-complete deprotonation of 1,2,4-H3BTC in 1 and 2,which is coincident with the results of crystallographic structural analysis.On the other hand,the thermogravimetric analyses(TGA)of compounds 1 and 2 were performed and the results are shown in Fig.5.For complex 1,no obvious weight loss was observed before 350℃,and above this temperature,the structure decomposed due to the liberation of the organic ligands.For complex 2,the first weight loss of 10.0%between 25 and 215℃ indicates the loss of free and coordinated water molecules(calcd.9.17%), and the residue remains unchanged until about 340°C, then a rapid weight loss was detected,which is attributed to the decomposition of the complex.

Fig.5 TG curves of compounds 1 and 2
[1]Kitagawa S,Kitaura R,Noro S.Angew.Chem.Int.Ed.,2004, 43:2334-2375
[2]WANG Cui-Juan(王萃娟),ZHOU Xian-Li(周先礼),REN Ping-Di(任平弟),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26:1900-1903
[3]Zhang J P,Lin Y Y,Huang X C,et al.Chem.Commun.,2005: 1258-1260
[4]SU Yan-Hui(苏延辉),CAO Deng-Ke(曹登科),ZHENG Li-Min(郑丽敏).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao), 2010,26:1617-1622
[5]BAI Zheng-Shuai(白正帅),CHEN Man-Sheng(陈满生), CHEN Shui-Sheng(陈水生),et al.Chinese J.Inorg.Chem. (Wuji Huaxue Xuebao),2009,25:402-406
[6]Férey G.Chem.Soc.Rev.,2008,37:191-214
[7]Zhang X M,Hao Z M,Zhang W X,et al.Angew.Chem.Int. Ed.,2007,46:3456-3459
[8]Zhao X Q,Zhao B,Shi W,et al.CrystEngComm,2009,11: 1261-1269
[9]Chen M S,Bai Z S,Okamura T-a,et al.CrystEngComm,2010, 12:1935-1944
[10]ZHANG Ping(张平),MENG Xiang-Guang(孟祥光),DU Juan (杜娟),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao), 2009,25:1371-1374
[11]Ghosh S K,Zhang J P,Kitagawa S.Angew.Chem.Int.Ed., 2007,46:7965-7968
[12]Wang X L,Qin C,Wang E B,et al.Angew.Chem.Int.Ed., 2005,44:5824-5827
[13]Zhang Z H,Okamura T-a,Hasegawa Y,et al.Inorg.Chem., 2005,44:6219-6227
[14]Zhu H F,Fan J,Okamura T-a,et al.Inorg.Chem.,2006,45: 3941-3948
[15]Sudik A C,Cté A P,Wong-Foy A G,et al.Angew.Chem.Int. Ed.,2006,45:2528-2531
[16]Wang R H,Zhou Y F,Sun Y Q,et al.Cryst.Growth Des., 2005,5:251-256
[17]Chen S S,Fan J,Okamura,T-a,et al.Cryst.Growth Des., 2010,10:812-822
[18]Chu Q,Liu G X,Huang Y Q,et al.Dalton Trans.,2007:4302-4311
[19]Liu G X,Huang Y Q,Chu Q,et al.Cryst.Growth Des.,2008, 8:3233-3245
[20]Fan J,Gan L,Kawaguchi H,et al.Chem.-Eur.J.,2003,9: 3965-3973
[21]Altomare A,Cascarano G,Giacovazzo C,et al.J.Appl.Cryst., 1994,27:435-436
[22]Beurskens P T,Admiraal G,Beurskens G,et al.The DIRFID-99 Program System,Technical Report of the Crystallography Laboratory,University of Nijmegen,Nijmegen,The Netherlands,1999.
[23]CrystalStructure 3.8:Crystal Structure Analysis Package, Rigaku and Rigaku Americas,9009 New Trails Dr.The Woodlands TX 77381 USA,2000-2007.
[24]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement,University of Göttingen,Göttingen,Germany, 1997.
[25]Addison A W,Rao T N,Reedijk J,et al.J.Chem.Soc. Dalton Trans.,1984:1349-1356
刚性配体1,3,5-三(1-咪唑基)苯与1,2,4-苯三甲酸构筑的金属配合物:水热合成及晶体结构
苏 志1吕高超1岗村高明2陈满生1陈水生1孙为银*,1
(1南京大学化学化工学院,配位化学国家重点实验室,配位化学研究所,南京 210093)
(2大阪大学理学研究科高分子学科,豐中市 560-0043,日本)
由水热法合成了 2个配合物[Co(1,2,4-HBTC)(tib)](1)和[Ni4(1,2,4-BTC)2(tib)4(H2O)2]·(1,2,4-HBTC)·9H2O(2)(1,2,4-H3BTC=1,2,4-苯三甲酸,tib=1,3,5-三(1-咪唑基)苯),并用元素分析、红外光谱、X-射线单晶衍射及热重分析等对其进行了表征。晶体结构分析结果表明:配合物1是由Co(Ⅱ)和tib连接形成的二维层状结构,1,2,4-HBTC2-作为端基配体与Co(Ⅱ)配位,而配合物2是通过1,2,4-BTC3-连接[Ni(tib)]2+二维网形成最终的二维多层结构,这2个化合物最终均被氢键连接形成三维超分子结构。
1,2,4-苯三甲酸;1,3,5-三(1-咪唑基)苯;晶体结构;水热合成
O614.81+2;O614.81+3
A
1001-4861(2011)05-0971-06
2010-10-09。收修改稿日期:2011-01-07。
国家自然科学基金重点项目(No.20731004)资助。
*通讯联系人。E-mail:sunwy@nju.edu.cn;会员登记号:S060015599P。
猜你喜欢
杂志排行
无机化学学报的其它文章
- Influence of Carbon Nanotube Content on Microstructures and Mechanical Properties of Carbon/Carbon Composite
- Syntheses,Crystal Structures and Electrochemical Properties of Cobalt and Nickel Complexes Containing Imidazole Ligand
- Hydrothermal Syntheses,Crystal Structures and Luminescent Properties of Binuclear Zn(Ⅱ)and Co(Ⅱ)Complexes Assembled by 4-Carboxymethoxy Phenylacetic Acid
- Thermal Stability and Antibacterial Activity of Phosphonium Salts Pillared Layered α-Zirconium Phosphates
- Synthesis of Porous Hydromagnesite Microspheres with Rosette-Like Morphology
- Effect of Synthesis Temperature on Structure and Ceramization Process of Polyaluminasilazanes
