口蹄疫病毒非结构蛋白3AB的原核表达及应用
2011-09-07邵军军常惠芸丛国正独军政高闪电
邵军军,常惠芸,林 彤,丛国正,独军政,高闪电
中国农业科学院兰州兽医研究所 家畜疫病病原生物学国家重点实验室 农业部畜禽病毒学重点开放实验室,兰州 730046
口蹄疫病毒非结构蛋白3AB的原核表达及应用
邵军军,常惠芸,林 彤,丛国正,独军政,高闪电
中国农业科学院兰州兽医研究所 家畜疫病病原生物学国家重点实验室 农业部畜禽病毒学重点开放实验室,兰州 730046
旨在建立一种检测口蹄疫病毒非结构蛋白抗体的敏感、特异的ELISA方法。克隆、表达了口蹄疫病毒非结构蛋白3AB基因,原核表达的重组蛋白经亲和层析法纯化及Western blotting鉴定后作为包被抗原,建立检测口蹄疫病毒非结构蛋白抗体的3AB间接ELISA方法,通过与商品化试剂盒3ABC-ELISA的比对试验对其进行评价。结果显示,重组蛋白3AB以包涵体形式表达;能与口蹄疫病毒感染血清发生特异性反应,而不能与疫苗免疫动物血清发生反应;在检测田间样品时,与3ABC-ELISA具有同样的特异性和敏感性 (P>0.05)。因此,认为重组蛋白3AB是一种十分有用的分子标记物,能有效区分口蹄疫病毒感染动物和疫苗免疫动物。
口蹄疫病毒,蛋白,反转录聚合酶链反应 (RT-PCR),酶联免疫反应 (ELISA)
Keywords: foot-and-mouth disease virus, protein, RT-PCR, ELISA
Introduction
Foot-and-mouth disease (FMD) is a highly contagious and the most important animal disease caused by foot-and-mouth disease virus (FMDV), which is a RNA virus belonging to the Aphthovirus genus within the family Picornaviridae and have seven distinct serotypes (O, A, C, SAT1-3 and Asia 1) and numerous subtypes[1]. Once outbreak of disease will severely impact livestock industry and cause huge economic losses on affected courntries. Therefore, vaccination of the susceptible animals as one of the major strategies is extensively used to control and prevent occures of FMD in the developing countries where the disease occur frequently. However, it has become a new difficulty problem that how to distinguish infected from vaccinated animals in herds after immunization. Vaccinated animals principally produce antibodies against structural proteins due to vaccines are formulated with the partially purified killed virus, while FMDV infection induce all antibodies to both the structural proteins (SP) and nonstructural proteins (NSPs). So a serological survey for antibodies to NSPs of FMDV can be used to differentiate infected from vaccinated animals in a herd[2-3]. In addition, the identification of infected animals may easily monitor animal health in a herd, and help to the control and eradication of disease in courntries where FMD is endemic and is controlled by preventive vaccination. Several ELISA based on NSPs 3ABC region have been developed to differentiate infected and vaccinated animals in herd[2-6].
In this study, a promising alternative diagnostic reagent 3AB was prepared and used as a coating antigen. An indirect ELISA was established and its availability were evaluated. The data showed that this method could be potentially used to differentiate FMDV infected from vaccinated animals.
1 Materials and methods
1.1 Sera
Sera from naive animals: Total of 220 serum samples were collected from cattle without antibodies to both SP and NSPs. Sera from vaccinated animals: 166 serum samples from cattle were collected at 3 weeks after vaccination. 40 infected sera were collected at 21 days post-infection from catlle infected experimentally with FMDV.
1.2 Expression and characterization of fusion protein 3AB
The 3AB gene of FMDV O/China/99 isolate was expressed in E. coli expression system as a recombinant protein attached to His-tag using the pET-30a(+) expression system (Novagen). The recombinant protein 3AB was purified by Ni-NTA His·Bind Resins (Novagen). The target protein was eluted and its purity was determined by SDS-PAGE. The antigenicity of the recombinant protein 3AB was confirmed by Western blotting with a positive serum from cattle infected with FMDV. The purified protein was quantified by Bradford assay (Pierce, Bonn, Germany) and stored at −20 °C until use.
1.3 Procedure of 3AB-indirect ELSIA
Indirect ELISA was performed as discribed previously and OIE manual of diagonostic tests and vaccines for terrestrial animal (version 2004) and made some modifications slightly. Briefly, the microplates were coated with a predetermined dilution (2.0 μg/mL) of the recombinant protein 3AB in the carbonate buffer (pH 9.6) over night at 4 °C. After washing with the phosphate buffered saline (PBS) supplemented with 0.05% Tween-20 (PBST), 96-well microplate was blocked with 5% (W/V) BSA (Sigma) for 2 hours at 37 °C, and then thoroughly washed with PBST. Test sera were diluted 1:20 in blocking buffer, and incubated for 30 min at 37 °C. After five washes, the optimized concentration of rabbit anti-species antibodies conjugated horse radish peroxidase (Sigma) was added at 37 °C for 30 min. After washing enough, 100 μL of reaction substrate mixture (TMB/H2O2) per well was added and color was developed at 37 °C in a dark box for about 10-15 min, and the reaction was then stopped by 2.0 mol/L H2SO4. The optical density (OD) values were read at 450 nm by a microplate reader (Bio-Rad).
1.4 Establishment of cut-off value
To establish the cut-off value of 3AB-I-ELISA, totalof 441 sera from naive (n=220), vaccinated (n=166) and infected animals (n=40) were tested. The frequency distribution (95%±2sd) of positive and negative samples was established by testing panels of sera with 3AB indirect ELISA. The S/P value of the ELISA results from each serum sample was calculated. The S/P value was the value of sample absorbance minus the negative control absorbance divided by the value of positive minus negative control absorbance.
2 Results
2.1 Characterization of the positive recombinants
The positive recombinants were identified with restriction endonuclease digestion, PCR amplification and sequencing. The results showed that a band of 672 bp corresponding to the 3AB coding frame in the electrophoresis gel (Fig. 1). Sequencing verified that 3AB gene was inserted correctly into the expression plasmid. No any mutation was found in the 3AB gene coding region.
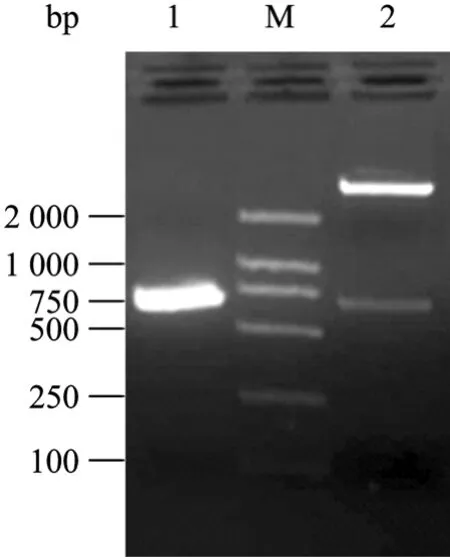
Fig.1 Indentification of recombinant expression plasmid with PCR and enzyme digestion with BamH I and Hind Ⅲ. 1: PCR product of 3AB gene; M: DNA molecular marker 2 000; 2: recombinant plasmid by enzyme digestion.
2.2 Expression and characterization of fusion protein 3AB
The antigenicity of the purified recombinant protein 3AB was analysed and confirmed in SDS-PAGE (Fig. 2). The estimated size of the NSP was the expected range according to amino acid sequence deduced. The Western blotting results showed that the recombinant protein 3AB was specially recognized by sera from the FMDV infected animal, but no reactivity with the sera from vaccinated and healthy animals (Fig. 3). The recombinant protein 3AB was in the formation of insoluble, namely inclusion bodies, and the amount of recombinat protein expression is about over 30% of total proteins (data not shown).
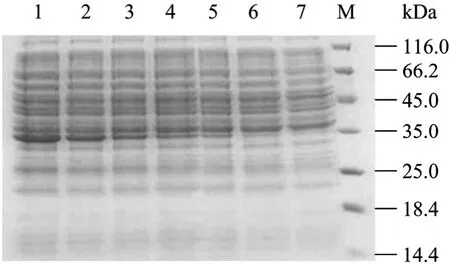
Fig.2 Analysis of 3AB fusion protein by SDS-PAGE. 1: 6 h; 2: 5 h; 3: 4 h; 4: 3 h; 5: 2 h; 6: 1 h; 7: 0 h; M: standard protein marker.
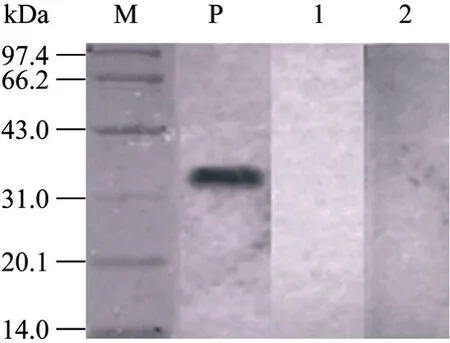
Fig.3 Analysis of 3AB fusion protein by Western blotting. M: standard protein marker; P: serum from FMDV infected animal; 1: serum from vaccinated animal; 2: negative control.
2.3 Establishement of cut-off value
Frequency distributions of S/P ratios were performed for the antibody from examination of 386 negative and 40 FMDV infected sera in the profile ELISA. For 3AB, there was no overlapping of positive and negative populations. To simplify interpretation, it was decided to set the opitimized cut-off so as to provide the best balance of sensitivity (100%) and specificity (98.9%). On this basis, S/P ratios 0.3 was considered positive.
2.4 Comparison of the specificity of 3AB and 3ABC
To test whether 3AB recombinant protein was suitable to distinguish vaccinated from infected animals, the antigenicity of purified 3AB and 3ABC was compared by detection of the field samples, respectively. 124 serum samples were collected from FMDV infected cattle and experimentally infected, 360 sera from cattle vaccinated with a commercial killed vaccine (O/A bivalent vaccine), 150 sera fromnaive cattle. The results showed that 124 infected sera were the positive for NSPs of FMDV antibodies detected by two assays. 150 sera from naive cattle were negative tested by two ELISA systems. However, 13 out of 360 vaccinated sera were positive determined by 3ABC-ELISA, 11 samples in 360 vaccinated sera were positive showed by 3AB-I-ELISA (Table 1). Accordingly, the specificity [(positive sera+negative sera)/total tested sera×100%] of the 3AB-I-ELISA and 3ABC-ELISA were 98.3% (623/634) and 97.9% (621/634) respectively. The statistic analysis showed that two kits were no significant difference for detection of antibodies to NSPs of FMDV (х2-test, P>0.05).
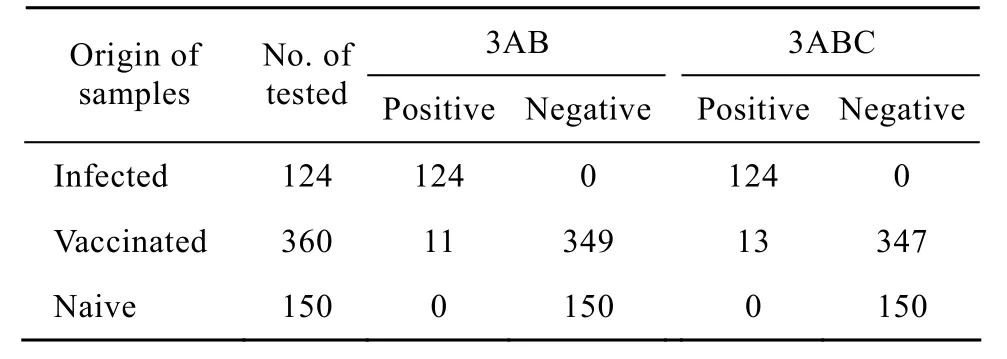
Table1 Comparison of the 3AB-I-ELISA and 3ABC-ELISA in detection of the field samples
3 Discussion
FMD is the most important viral infectious disease of the cloven-hoofed animals including cattle, pigs, sheep and goats. In recent years, outbreaks of disease occurred frequently worldwide severely impact the livestock industry. Vaccination of animal is one of the most important policies for control and preventtion of disease, which was extensively used to control and prevent FMD in many of developing countries where disease is endemic. However, some vaccinated ruminant may become the persistent infectious animals termed “carrier”, and require discrimination infected from vaccinated animals in the international trade. So, it is very important for developing a feasible diagnostic method to distinguish infected from vaccinated animals in a herd and is still necessary for early warning of FMD outbreak and medical inspection in export and import of livestock and their flesh products.
So far, the several NSPs-ELISA kits have been developed to distinguish infected animals from those that have been vaccinated[4-6]. The NSPs of FMDV from recombinant proteins in a prokaryotic and/or eukaryotic system had been used to detection of antibodies against the NSPs of FMDV. Large number of tests have confirmed that detection of antibodies to the 3ABC or/and other’s NSPs were the most successful assay to distinguish FMDV infected animals from vaccinated animals in a herds[7-8]. Though, the variations in sensitivity and specificity were reported in many types of ELISAs as well as NSP antigens[4,9-12], however, the recombinant NSPs of FMDV and their applications as antigen in ELISA were still shown to be promising diagnostic methods that can identify infected animals with FMDV in herds[4,9,11]. Nanni et al reported that the target protein expression amount was up to 30 mg per liter of bacteria culture, following a 3AB-ELISA kit to detection of NSP antibodies based on 3AB recombinant antigens has been developed. The tested results showed that their kit was not only a simple and fast diagnostic method, but also have the higher specificity and sensitivity compared with commercial kits. In addition, previous reports also indicated that 3AB protein was more sensitivity than 3ABC in detection of antibodies to NSPs of FMDV, and the different kits have the different sensitivity and specificity in detecion of antibodies to NSPs of FMDV[13-15]. Therefore, it is advisable to employ the combination of the different kits, which could increase the sensitivity and specificity in the large-scale screening in the nationwide surveillance and avoid the false negative and false positive.
In this study, to develop a reliable diagnostic protein to enhance the capability of differentiation vaccinated from FMDV infected animals in a herd, 3AB gene of FMDV were cloned and expressed successfully in E. coli BL21 (DE3). Western blotting showed that the recombinant protein 3AB was able to be effectively recognized by the positive sera from cattle infected experimentally with FMDV. 3AB indirect ELISA can be used for distinguishing infected from vaccinated animals. To validate the performance of 3AB-I-ELISA, a commercial available 3ABC-ELISA kit was used to make a comparison. The analytical results showed that specificity and sensitivity were no significant differences between the two ELISAs. So we speculated that the 3AB-I-ELISA may be used to detect antibodies against NSPs of FMDV from variousserotypes of FMDV due to the nonstructral protein region of FMDV were highly conserved among all seven FMDV serotypes. The data showed that 3AB-I-ELISA is a feasible diagnostic method and could specifically detect the antibodies induced by FMDV infection irrespective of their serotypes.
REFERENCES
[1] Doel TR. FMD vaccines. Virus Res, 2003, 91(1): 81−89.
[2] Clavijo A, Zhou EM, Hole K, et al. Development and use of a biotinylated 3ABC recombinant protein in a solid-phase competitive ELISA for the detection of antibodies against foot-and-mouth disease virus. J Virol Methods, 2004, 120(2): 217−227.
[3] Lu Z, Cao Y, Guo J, et al. Development and validation of a 3ABC indirect ELISA for differentiation of foot-and-mouth disease virus infected from vaccinated animals. Vet Microb, 2007, 125(1/2): 157−169.
[4] Bergmann IE, Malirat V, Neitzert E, et al. Improvement of a serodiagnostic strategy for foot-and-mouth disease virus surveillance in cattle under systematic vaccination: a combined system of an indirect ELISA-3ABC with an enzyme-linked immunoelectrotransfer blot assay. Arch Virol, 2000, 145(3): 473−489.
[5] Shen F, Chen PD, Walfield AM, et al. Differentiation of convalescent animals from those vaccinated against foot-and-mouth disease by a peptide ELISA. Vaccine, 1999, 17(23/24): 3039−3049.
[6] Sorensen KJ, Madsen KG, Madsen ES, et al. Differentiation of infection from vaccination in footandmouth disease by the detection of antibodies to the non-structural proteins 3D, 3AB, and 3ABC in ELISA using antigens expressed in baculovirus. Arch Virol, 1998, 143(8): 1461−1476.
[7] Chung WB, Sorensen KJ, Liao PC, et al. Differentiation of foot-and-mouth disease virus-infected from vaccinated pigs by enzyme-linked immunosorbent assay using nonstructural protein 3AB as the antigen and application to an eradication program. J Clin Microb, 40(8): 2843−2848.
[8] Mackay DKJ, Forsyth MA, Davies PR, et al. Differentiating infection from vaccination in foot-andmouth disease using a panel of recombinant, nonstructural proteins in ELISA. Vaccine, 1998, 16(5): 446−459.
[9] De Diego M, Brocchi E, Mackay D, et al. The non-capsideal polyprotein 3ABC of foot-and-mouth disease virus as a diagnostic antigen in ELISA to differentiate infected from vaccinated cattle. Arch Virol, 1997, 142(10): 2021−33.
[10] Lubroth J, Brown F. Identification of native foot-andmouth disease virus nonstructural protein 2C as a serological indicator to differentiate infected from vaccinated livestock. Res Vet Sci, 1995, 59(1): 70−78.
[11] Meyer RF, Babcock GD, Newman JF, et al. Baculovirus expressed 2C of foot-and-mouth disease virus has the potential for differentiating convalescent from vaccinated animals. J Virol Methods, 1997, 65(1): 33−43.
[12] Silberstein E, Kaplan G, Taboga O, et al. Foot and mouth disease virus infected but not vaccinated cattle develop antibodies against recombinant 3AB nonstructural protein. Arch Virol, 1997, 142(2): 795−805.
[13] Lee F, Lin YL, Jong MH. Comparison of ELISA for the detection of porcine serum antibodies to non-structural proteins of foot-and-mouth disease virus. J Virol Methods, 2004, 116(2): 155−159.
[14] Brocchi E, Bergmann IE, Dekker A, et al. Comparative evaluation of six ELISAs for the detection of antibodies to the non-structural proteins of foot-and-mouth disease virus. Vaccine, 2006, 24(47/48): 6966−6979.
[15] He C, Wang H, Wei H, et al. A recombinant truncated FMDV 3AB protein used to better distinguish between infected and vaccinated cattle. Vaccine, 28(19): 3435−3459.
Expression and utilization of 3AB nonstructural protein of foot-and-mouth disease virus in Escherichia coli
Junjun Shao, Huiyun Chang, Tong Lin, Guozheng Cong, Junzheng Du, and Shandian Gao
Key Laboratory of Animal Virology of Minister of Agriculture, State Key Laboratory of Veterinary Etiological Biology, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Lanzhou 730046, China
To develop a sensitive and specific ELISA for detection of antibodies to the nonstructural protein of FMDV. We cloned and expressed FMDV nonstructural protein 3AB in Escherichia coli expression system. The recombinant protein 3AB was purified with Ni-NTA His·Bind Resins and characterized by Western blotting. An indirect ELISA based on purified protein 3AB as a coating antigen was established. The specificity and sensitivity of this assay were evaluated by comparison with a commercial 3ABC-ELISA kit in detecion of serum samples. The results showed that the recombinant protein 3AB was expressed as a formation of inclusion bodies in Escherichia coli. The purified protein could specificially react with FMDV infection antibodies in Western blotting assay, but no reaction with the immune antibodies induced with vaccine. Two assays were no significant differences in specificity and sensitivity for detection of field samples (P>0.05). Therefore, we speculated that the recombinant protein 3AB is a promising molecular marker, which may effectively differentiate FMD-infected from vaccinated animals in a herd.
May 14, 2010; Accepted: July 27, 2010
Huiyun Chang. Tel/Fax: +86-931-8342359; E-mail: changhuiyun@126.com
Supported by: Key Program of Science and Technology of Gansu Province (No. 092NKDA030), National High Technology Research and Development Program of China (863 Program) (No. 2006AA10A204).
甘肃省科技重大专项 (No. 092NKDA030),国家高技术研究发展计划 (863计划) (No. 2006AA10A204) 资助。
