结晶和去润湿竞争条件下分子结构对超薄膜表面形貌的影响
2010-12-11袁伟忠张凤波袁金颖谢续明洪啸吟
袁伟忠 张凤波 袁金颖 谢续明 洪啸吟
(1清华大学化学系,有机光电子与分子工程教育部重点实验室,北京 100084;2同济大学材料科学与工程学院,纳米与生物高分子材料研究所,上海 200092;3清华大学化学工程系,北京 100084)
结晶和去润湿竞争条件下分子结构对超薄膜表面形貌的影响
袁伟忠1,2,§张凤波3,§袁金颖1,*谢续明3洪啸吟1
(1清华大学化学系,有机光电子与分子工程教育部重点实验室,北京 100084;2同济大学材料科学与工程学院,纳米与生物高分子材料研究所,上海 200092;3清华大学化学工程系,北京 100084)
将星形支化结构的聚己内酯,包括六臂星形聚己内酯(HPCL)和树枝状星形聚己内酯(DPCL),以及线形聚己内酯(LPCL)室温旋涂于云母片上,通过原子力显微镜(AFM)观察分子结构对星形支化聚己内酯超薄膜的润湿-去润湿性质的影响.在旋涂过程中,薄膜的形成受去润湿和结晶竞争的控制.差示扫描量热(DSC)测试结果表明,当相对分子质量相同时,结晶性的顺序是:DPCL最弱,HPCL次弱,LPCL最强.依据分子结构和相对分子质量的影响,即去润湿和结晶竞争的结果,LPCL、HPCL和DPCL的超薄膜表现出不同的表面形态,包括尺寸不同的完整的球晶、开口的球晶、树枝状片、分散的颗粒.
润湿;分子结构;去润湿;结晶;超薄膜
Theultrathinfilm(less than 100 nm in thickness)morphologies of polymers have attracted increasing interest due to their scientific importance and potential applications in many fields[1-6].The morphology of polymer ultrathin films is affected by various factors,such as polymer structure,substrate surface properties, preparation conditions,and thermal history,among which the polymerstructureisoneofthemostimportantfactors[7-10].Themolecular structure of polymer also affects the wetting-dewetting behavior of polymer ultrathin film[11].A contiguous ultrathin film of polymer on a substrate will tend to minimize its free energy by exposing the lower surface energy material to environment. If the surface energy of the coating,γc,is lower than that of the underlying substrate,γs,the film is stable.However,if γcis higher than γs,the system will reduce its free energy by creating holes in the coating.This process is usually referred as dewetting.The stability of the coating is expressed in terms of a spreading coefficient,S,
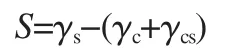
where γcsis the coating/substrate interfacial energy.If S is positive,the coating will wet the substrate and the film will be stable.If S is negative,the coating will dewet the substrate and the contact angle for the coating material on the substrate will be much greater than zero[12].In addition to the surface and interfacial energy,the film dewetting will depend on the film thickness. In films thinner than 100 nm(ultrathin film),attractive van der Waals forces dominate,causing the thin film to rapture spontaneously.Reiter et al.[13]reported the spontaneous dewetting process of thin polystyrene films on silicon substrates during thermal annealing above the glass transition temperature when their original thickness is less than 100 nm.Composto et al.[14]found that the stability of polystyrene films could be improved by blending with poly(styrene-b-methyl methacrylate)copolymer on silicon oxide substrate.For crystalline or semicrystalline polymer,the wetting-dewetting process of ultrathin film is more complicated.Namely,the morphology formation and the wettingdewetting process are ruled by two competing rate processes, crystallization of crystalline or semicrystalline polymer and dewetting.Xu et al.[15]reported the effect of substrate surface on dewetting behavior and chain orientation of symmetrical semicrystalline oxyethylene/oxybutylene block copolymer thin films and found that the silicon,modified silicon,and mica substrate surfaces showed different influence on the wetting-dewetting behavior of block copolymers.
Poly(ε-caprolactone)(PCL)is one of the most important aliphatic polyesters and considerable interests exist in it due to its biodegradability,biocompatibility,and good drug permeability[16-20]. Furthermore,PCL belongs to semicrystalline polymer and can be considered as an alternative model for the study of polymer crystallization,with a crystal structure very close to that of polyethylene,the model semicrystalline polymer[21].Kressler et al.[22]investigated the morphology formation and dewetting of thin films of linear PCL on glass substrate.Depending on the concentration of the solutions,PCL films completely covered the substrate,open spherulites were formed,or for very dilute solutions,PCL formed dispersed droplets on the substrate. Prud′homme et al.[21]investigated linear PCL crystal growth in ultrathin films by atomic force microscopy(AFM)and found that PCL ultrathin films crystallized with lamellae oriented flat on.
Until now,the effect of molecular structure on wettingdewetting behavior of star-branched PCL ultrathin films has not been investigated.In fact,the star-branched PCL presents different crystallization behavior from that of the linear PCL and will reveal unique wetting-dewetting behavior during the competing rate process of crystallization of star-branched PCL and dewetting.Therefore,in this work,star-branched PCL,including starshaped PCL and dendritic PCL with different relative molecular mass were prepared.As comparison,linear PCLs with differentrelativemolecularmasswereprepared,too.Thewettingdewetting processes of these PCL ultrathin films on mica substrate were investigated by atomic force microscopy(AFM).
1 Experimental
1.1 Materials
ε-Caprolactone(Acros Organic,USA)was purified with CaH2by vacuum distillation.Tin 2-ethylhexanoate(Sn(Oct)2,Aldrich, USA)was distilled under reduced pressure before use.Dipentaerythritol(Aldrich,USA)was dried at 100℃in vacuum for 24 h.The dendrimer polyester(Perstorp Specialty Chemicals,Sweden)(Mn=1747)was dried at 60℃in vacuo for 24 h before use. Benzyl alcohol,methylene chloride,chloroform,hexane,and tetrahydrofuran(THF)were local commercial products and dried over CaH2and distilled before use.
1.2 Characterization
1H NMR spectra were obtained from JOEL JNM-ECA300 NMR spectrometer with CDCl3as solvent.The chemical shifts were relative to tetramethylsilane at δ=0 for protons.The relative molecular mass and relative molecular mass distribution were measured on a Viscotek TDA 302 gel permeation chromatography equipped with two columns(GMHHR-H,M Mixed Bed).THF was used as eluent at a flow rate of 1 mL·min-1at 30℃.Differential scanning calorimetric(DSC)analysis was carried on a DSC 2910 thermal analysis system with a heating rate of 10℃·min-1from-30℃to 150℃under nitrogen atmosphere. Wide-angle X-ray diffraction(WAXRD)patterns of powder samples were obtained at room temperature on a Cu Kαradiation source using a Bruker AXS D8 Advance X-ray diffractometer (Bruker,Germany).The supplied voltage and current were set to 40 kV and 120 mA.Samples were exposed at a scanning rate of 2θ=4(°)·min-1between 2θ values from 1.5°to 60°.The crystalline morphologies of the polymers were observed using a SPM-9500J3 atomic force microscope(Shimadzu,Japan).The ultrathin films on transparent mica substrate were obtained from spincoating of polymer solution(0.8 g·L-1)and the thickness was about 15 nm.The spin-coating temperature was 25℃,and the rotation speed was 1000 r·min-1.After that the films were annealed at the same temperature for sufficient crystallization.
1.3 Syntheses of star-branched PCLs and linear PCL
In this study,star-branched PCL(hexa-armed star-shaped PCL and dendritic PCL)and linear PCL were synthesized through Sn(Oct)2catalyzedring-openingpolymerization(ROP)of ε-capro-lactone(CL)monomer with corresponding multifunctional initiators(dipentaerythritol and hydroxyl-terminated dendrimer polyester)and benzyl alcohol initiator(Scheme 1).A typical polymerization procedure was shown as follows:dendrimer polyester initiator(0.176 g,0.101 mmol),CL(6.2 g,54.3 mmol),a catalytic amount Sn(Oct)2,and a dried magnetic stirring bar were added into a fire-dried polymerization tube.The tube then connected to aSchlenkline,whereexhausting-refillingprocesses were repeated three times.The tube was immersed into an oil bath at 115℃under nitrogen atmosphere with vigorous stirring for 24 h.After cooling to room temperature,the resulting polymer was dissolved in chloroform and precipitated twice with hexane to afford the purified dendritic polymer(DPCL3).The purified polymer was dried in a vacuum oven until constant mass.
2 Results and discussion
2.1 Preparation of well-defined star-branched PCLs
Biodegradable aliphatic polyesters with well-defined architecture can be synthesized from ROP of lactides and/or lactones using a hydroxyl-terminated compound as initiator and Sn(Oct)2as catalyst.As shown in Table 1,Mnof the star-branched PCLs linearly increased with the monomer to the initiator,and the relative molecular mass distributions were very narrow(Mw/Mn≤1.18 for HPCL and Mw/Mn≤1.16 for DPCL);this indicated that the hydroxyl groups in dipentaerythritol and dendrimer surfaces were used as effective propagation centers.
2.2 DSC and WAXRD analysis
The thermal properties of the star-branched PCLs were investigated with DSC and compared with those of linear counterparts.Table 1 shows data for the melting point(Tm),enthalpy offusion(ΔH),anddegreeofcrystallinity(Xc)ofthestar-branched and linear PCLs.According to the data,the melting point and degree of crystallinity decreased with the increasing arm number of PCL,and they are in the order of DPCL<HPCL<LPCL,when the relative molecular mass of the polymers is similar to each other(suchassamplesofDPCL1,HPCL3,andLPCL2).Asshown in Table 1,Tmand Xcwere 44.8℃and 41.4%for DPCL1,55.1℃and 48.9%for HPCL3,whereas 60.8℃and 54.2%for LPCL2,although they possessed similar relative molecular mass; this was attributed to the crystalline imperfection due to the short chain length of star-branched PCL arms.Moreover,the branched structure of these polymers should make a contribution to the imperfection.With the increase in degree of branching of PCL molecule,the hindrance of chain movements increased, and the crystallizability of these polymers decreased.For PCL with similar relative molecular mass and different branches,Xcdecreased with increasing the number of arms,suggesting that the decrease of crystallinity of PCLs could be achieved by increasing the degree of branching.Moreover,Tmand Xcof starbranched PCL increased with the length of PCL arms.For example,Tmand Xcof DPCL2 were 49.1℃ and 45.0%,respectively,whereas Tmand Xcof DPCL3 were 54.8℃and 48.8%, respectively.For linear PCL,the increase of Tmand Xcwith the length of PCL was less obvious than that for the star-branched PCL;this should be attributed to the fact that the crystalline imperfection and steric hindrance of star-branched PCL were much more serious than those of linear PCL.

Table 1 Relative molecular mass and thermal properties of star-branched and linear PCLa
WAXD analysis was used to investigate the crystalline structure of star-branched and linear PCL.The results of LPCL2, HPCL2,and DPCL2 are shown in Fig.1.It could be seen that these polymers show intensive peaks at 21.6°,22.1°,and 23.9°, corresponding to the(110),(111),and(200)planes of the orthorhombic crystal form.As a result,the star-branched and linear PCLs presented the same crystalline structure,which suggested that the branched structure did not change the crystalline structure of PCL.
2.3 Wetting-dewetting processes of star-branched and linear PCL
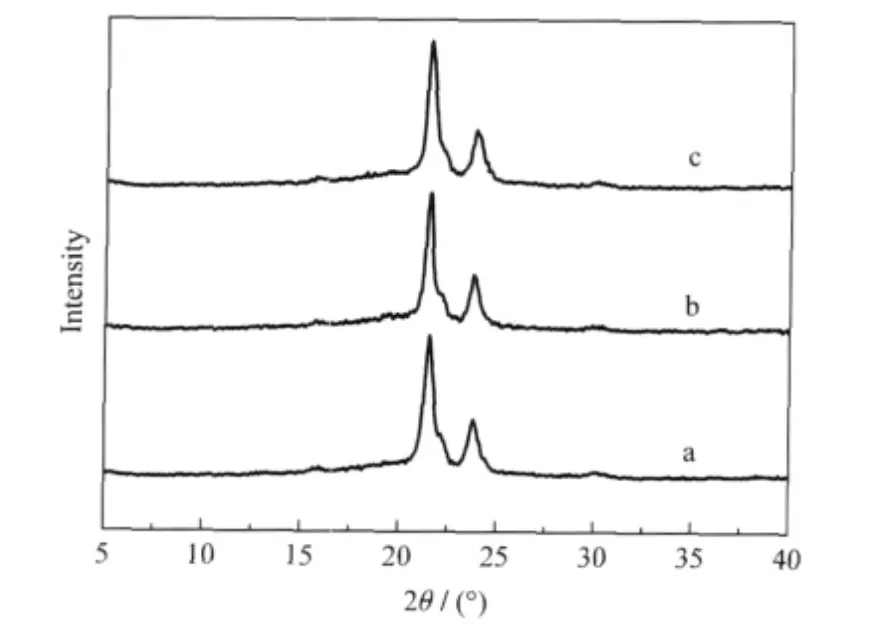
Fig.1 WAXRD patterns of samples of(a)LPCL2,(b) HPCL2,and(c)DPCL2
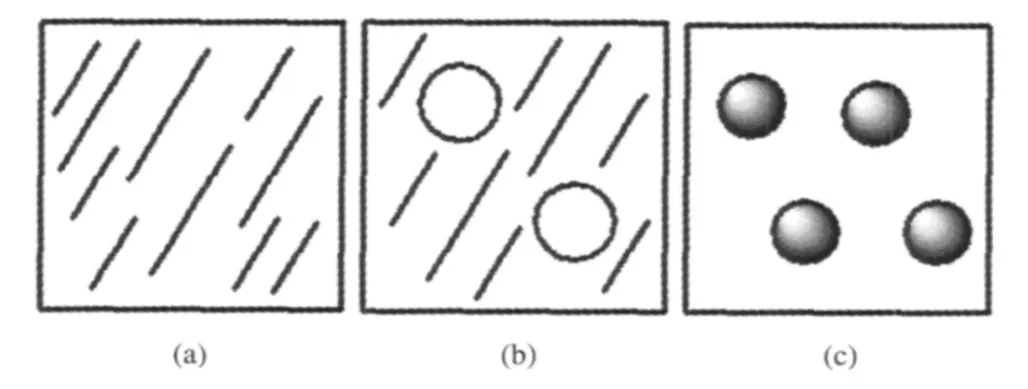
Fig.2 Schematic drawing of three kinds of surface morphology of ultrathin film(a)complete film-forming,(b)wetting-dewetting process, (c)complete dewetting process
The wetting-dewetting processes of LPCL,HPCL,and DPCL were investigated by AFM images.Ultrathin films of PCL (about 15 nm)were prepared by spin-coating process.The morphology formation of PCL is ruled by two competing rate processes,crystallization of PCL and dewetting.The dewetting process will occur due to the inherent instability of the ultrathin films.Therefore,competition of the two rate processes offers the possibility for formation of various structures.Fig.2 shows the schematic drawing of three kinds of surface morphology of ultrathin film.If crystallization(wetting process)surpasses dewetting process,perfect ultrathin film can form(Fig.2(a));if the rate of wetting process is equal to that of dewetting process,then imperfect film with holes forms(Fig.2(b));if dewetting process exceeds wetting process,polymer film will rupture and polymer solution shrinks to“spheres”(dispersed droplets)(Fig.2(c)).Fig.3 shows samples of AFM micrographs of LPCL(LPCL1,LPCL2, and LPCL3),HPCL(HPCL1,HPCL2,and HPCL3),and DPCL (DPCL1,DPCL2,and DPCL3).These measurements were carried out in the height mode.
To LPCL,it could be found that almost uniform“spheres”alongwithinthe image of LPCL1(Fig.3(a)).The formation of these“spheres”could be ascribed to shrinking of the ruptured polymer ultrathin film,which indicated that the dewetting process surpassed crystallization of PCL due to the low relative molecular mass of LPCL1.The size of these“spheres”was arranged from 1.8 to 3 μm.The presence of the sparse flat-on lamellae on the mica surface indicated that the crystalline growth of LPCL1 was still carried out,although the dewetting process was dominating.The formation of the almost perfect two-dimensional spherulites in LPCL2 showed that the rate of crystallization was much faster than that of dewetting(Fig.3(b)).In fact,according to the lamellae morphology,the lamellae tended to form the dendritic crystals due to the ultrathin film.However,the lamellae were enough compact due to the crystallizability of LPCL2, which led to the formation of two-dimensional spherulites.The obvious crystal boundary and the lamellae divergent growth were the evidence of the spherulites growth style.Moreover, hole nucleation process of spherulites indicated that the dewetting process still existed during the process of crystalline growth.As shown in Fig.4(a),the average thickness of lamellae was obtained from cross-section profile and about 10-12 nm, which confirmed the flat-on orientation of lamellae.However, the spherulites in LPCL3 were less perfect than those in LPCL2 and the open spherulites were formed owing to the diffusion process(Fig.3(c)),which might be attributed to the much larger relative molecular mass than that of LPCL2.The diffusion process was along with the crystalline growth of LPCL3 and the dewetting was dominating.Therefore,the density of lamellae in LPCL3 was less than that in LPCL2.

Fig.3 AFM height images of ultrathin films(10-15 nm)of(a)LPCL1,(b)LPCL2,(c)LPCL3,(d)HPCL1, (e)HPCL2,(f)HPCL3,(g)DPCL1,(h)DPCL2,and(i)DPCL3
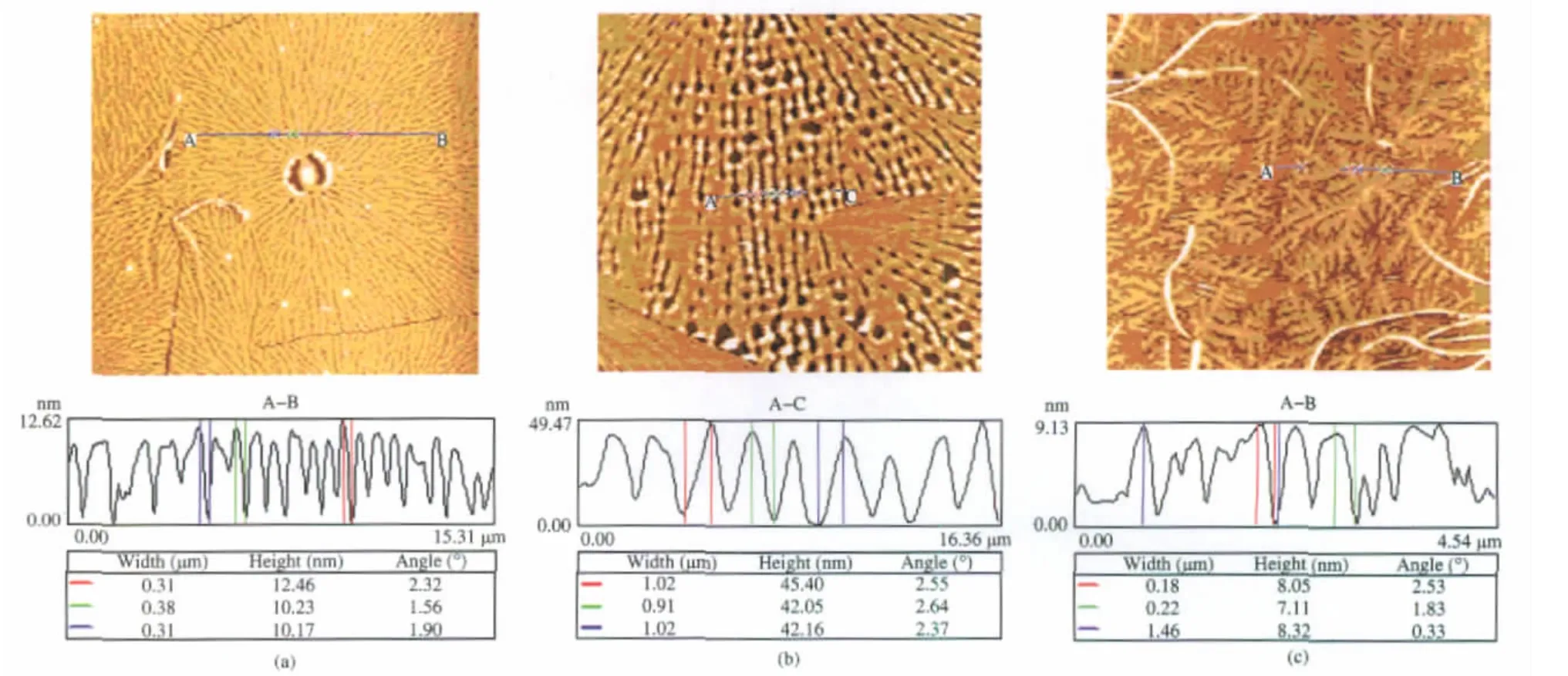
Fig.4 AFM images and their cross-sectional profiles of(a)LPCL2,(b)HPCL3,and(c)DPCL2
To HPCL,uniform small“spheres”arranged in the images of HPCL1 and HPCL2(Fig.3(d-e)).“Spheres”in HPCL2 were bigger than those in HPCL1 but less than those in LPCL1(size:100 nm in HPCL1 and 150 nm in HPCL2),which should be ascribed to the hexa-armed branched structure and low relative molecular mass of HPCL1 and HPCL2.The ultrathin films of HPCL1 and HPCL2 were more instable than that of LPCL1.The dewetting process was rapid and the films were ruptured to form polymer droplets and the crystallization process could not occur.In the image of HPCL3,open and imperfect spherulites formed(Fig.3 (f));this indicated that HPCL3 presented faster crystallization rate than dewetting,although the dewetting was still serious. Many holes could be found in the image,indicating that the dewetting led to the rupture of the ultrathin film.The lamella grew along with the zones of polymer solution.Therefore,the density of lamellae in HPCL3 was less than that in LPCL2,but the lamellae in HPCL3 were thicker than those in LPCL2.According to the cross-sectional profile(Fig.4(b)),the thickness of lamellae was about 42-45 nm,typical edge-on orientation of lamellae.As a result,the dewetting process in HPCL3 was stronger than that in LPCL2,although their relative molecular mass is almost the same,which should be attributed to the branched structure of HPCL3.
To DPCL,the morphologies were more complicated than those of LPCL and HPCL.Although the relative molecular mass of DPCL1 was similar to that of LPCL2,spherulites could not form in the ultrathin film of DPCL1(Fig.3(g)).The main reasons included the high branched structure of polymer and the comparative rapid rate of dewetting process.Therefore,the crystalline growth of polymer was seriously affected and some imperfect dendritic crystals formed.An interesting phenomenon that some curving lamellae formed along with imperfect dendritic crystals could be discovered.The growth of edge-on lamellae was relatively fast but the growing direction of lamellae was restricted.In addition,the presence of many amorphous regions in ultrathin film restricted the growing direction of edgeon lamellae.Therefore,the edge-on lamellae did not present linear but long curving structure and was as the center of the imperfect flat-on dendritic crystals.Many daughter flat-on lamellae nucleated along the edge-on lamellae sides.Another interesting phenomenon was that there were more flat-on lamellae growing outside of the curving lamellae,which should be attributed to theenough space and“materials”for the flat-on lamellae to grow. The dendritic morphologies in Fig.3(g,f)should formed via a diffusion-limited aggregation(DLA)mechanism,which is frequently observed in crystallization of PEO ultrathin film[24].Along with the increase of length of PCL arms,the crystallizability of PCL increased and dewetting process decreased.The comparatively perfect dendritic crystals formed in DPCL2(Fig.3(h)).The formation of dendritic crystals demonstrated that the growth of lamellae was restricted by the branched structure of polymer and the dewetting process.In Fig.4(c),the thickness of lamellae was about 7-8 nm,thinner than that of lamellae in LPCL2.In LPCL, the dendritic lamellae could be observed when the thickness of ultrathin film was 6 nm.When the thickness of ultrathin film increased to 15 nm,only spherulites could be observed,as reported by Prud′homme[21].All these indicated that the DPCL present poor crystallizability than linear PCL.The formation of more perfect dendritic crystals in DPCL2 than those in DPCL1 indicated that PCL chains of DPCL2 presented better crystallizability than those of DPCL1.To DPCL3,the crystallizability of PCL increased evidently though the dewetting process was still obvious(Fig.3(i)).In some regions,comparatively perfect spherulites could be discovered in DPCL3 though the degree of perfection of spherulites was poorer than that in LPCL2.Around the spherulites,many holes could be observed,which should be ascribed to the dewetting process.
3 Conclusions
The wetting-dewetting behaviors of ultrathin films of starbranched PCLs were investigated.During the spin-coating process,film formation is ruled by competition of dewetting and crystallization.Due to the unique structure of star-branched PCLs,ultrathin films form perfect spherulites,open spherulite, dendritic lamellae,and dispersed droplets with different sizes, which indicate that the molecular structure of star-branched PCLs has obvious effect on the wetting-dewetting behavior of ultrathin films.The investigation of the properties of ultrathin film of star-branched PCL can extend to other crystallizable biodegradable polyesters,such as polylactide(PLA),polyglycolide(PGA),and their copolymers,which is important in biomedical field.
1 McEvoy,T.M.;Long,J.W.;Smith,T.J.;Stevenson,K.J. Langmuir,2006,22:4462
2 Wang,L.;Hong,S.;Hu,H.;Zhao,J.;Han,C.C.Langmuir,2007, 23:2304
3 Fredin,N.J.;Zhang,J.;Lynn,D.M.Langmuir,2007,23: 2273
4 Serghei,A.;Tress,M.;Kremer,F.Macromolecules,2006, 39:9385
5 Tiaw,K.S.;Teoh,S.H.;Chen,R.;Hong,M.H. Biomacromolecules,2007,8:807
6 Bodiguel,H.;Fretigny,C.Macromolecules,2007,40: 7291
7 Cao,T.;Wei,F.;Jiao,X.;Chen,J.;Liao,W.;Zhao,X.; Cao,W.Langmuir,2003,19:8127
8 Zimnitsky,D.;Jiang,C.;Xu,J.;Lin,Z.;Tsukruk,V.V. Langmuir,2007,23:4509
9 Such,G.K.;Quinn,J.F.;Quinn,A.;Tjipto,E.;Caruso,F. J.Am.Chem.Soc.,2006,128:9318
10 Singamaneni,S.;LeMieux,M.C.;Jiang,H.;Bunning,T. J.;Tsukruk,V.V.Chem.Mater.,2007,19:129
11 Ashley,K.M.;Raghavan,D.;Douglas,J.F.;Karim,A. Langmuir,2005,21:9518
12 Oslanec,R.;Costa,A.C.;Composto,R.J.;Vlcek,P. Macromolecules,2000,33:5505
13 Reiter,G.;Sharma,A.;Casoli,A.;David,M.O.;Khanna, R.;Auroy,P.Langmuir,1999,15:2551
14 Costa,A.C.;Geoghegan,M.;Vlcek,P.;Composto,R.J. Macromolecules,2003,36:9897
15 Xu,J.T.;Liang,G.D.;Fan,Z.Q.;Mai,S.M.;Ryan,A.J. Macromolecules,2006,39:5471
16 Li,S.;Garreau,H.;Pauvert,B.;McGrath,J.;Toniolo,A.; Vert,M.Biomacromolecules,2002,3:525
17 Yuan,W.Z.;Yuan,J.Y.;Zhang,F.B.;Xie,X.M. Biomacromolecules,2007,8:1101
18 Yuan,W.Z.;Yuan,J.Y.;Zhang,F.B.;Xie,X.M.;Pan,C.Y. Macromolecules,2007,40:9094
19 Yuan,W.Z.;Yuan,J.Y.;Zhou,M.;Pan,C.Y.J.Polym. Sci.Pol.Chem.,2008,46:2788
20 Yan,Q.;Yuan,J.Y.;Zhang,F.B.;Sui,X.F.;Xie,X.M.; Yin,Y.W.;Wang,S.F.;Wei,Y.Biomacromolecules,2009,10: 2033
21 Mareau,V.H.;Prud′homme,R.E.Macromolecules,2005,38: 398
22 Kressler,J.;Wang,C.;Kammer,H.W.Langmuir,1997, 13:4407
23 Yuan,W.Z.;Yuan,J.Y.;Zhou,M.;Sui,X.F.J.Polym. Sci.Pol.Chem.,2006,44:6575
24 Zhai,X.M.;Wang,W.;Zhang,G.L.;He,B.L. Macromolecules,2006,39:324
December 8,2009;Revised:February 5,2010;Published on Web:March 5,2010.
Effect of Molecular Structure on the Surface Morphology of Ultrathin Films by Competing Crystallization and Dewetting Processes
YUAN Wei-Zhong1,2,§ZHANG Feng-Bo3,§YUAN Jin-Ying1,*XIE Xu-Ming3HONG Xiao-Yin1
(1Key Laboratory of Organic Optoelectronics&Molecular Engineering of the Ministry of Education,Department of Chemistry, Tsinghua University,Beijing 100084,P.R.China;2Institute of Nano and Bio-Polymeric Materials,School of Material Science and Engineering,Tongji University,Shanghai 200092,P.R.China;3Department of Chemical Engineering,Tsinghua University, Beijing 100084,P.R.China)
Star-branched poly(ε-caprolactone)(PCL)including hexa-armed star-shaped PCL(HPCL),dendritic PCL (DPCL),and linear PCL(LPCL)were spin-coated onto mica substrates at room temperature.The effect of molecular structureonthewetting-dewettingbehaviorofultrathinfilm(about15nm)wasinvestigatedwithatomicforcemicroscopy (AFM).During spin-coating,film formation is governed by competing dewetting and crystallization processes. According to differential scanning calorimetry(DSC),the crystallizability of DPCL is weaker than that of HPCL which is weaker than that of LPCL,when they have the same relative molecular mass.Depending on the molecular structure and the relative molecular mass of PCL,the competition between crystallization and dewetting results in ultrathin films of LPCL,HPCL,and DPCL with surface morphologies such as perfect spherulites,open spherulites,dendritic lamellae,and dispersed droplets of different sizes.
Wetting; Molecular structure;Dewetting;Crystallization;Ultrathin film
*Corresponding author.Email:yuanjy@mail.tsinghua.edu.cn;Tel:+86-10-62783668.
§These authors contributed equally to this work.
The project was supported by the National Natural Science Foundation of China(20874056,20836004,20974058)and National Key Basic Research Program of China(973)(2009CB930602).
国家自然科学基金(20874056,20836004,20974058)和国家重点基础研究发展计划(973)(2009CB930602)资助项目
袁金颖,2000年7月至2002年6月在北京大学化学与分子工程学院高分子科学与工程系从事博士后研究.
O647
