一种新型纺锤状α-Fe2O3纳米晶的合成、表征及其表面性能
2010-03-06詹拥共陈启元尹周澜李莉莉蔡炳新
詹拥共 陈启元 尹周澜 李莉莉 蔡炳新,*
(1中南大学化学化工学院,长沙 410083; 2湖南大学化学化工学院,长沙 410082)
Transition metal oxides have attracted significant attention in a variety of fields because of their unique structures and properties[1-4].In particular,the performance can be significantly enhanced by downsizing to nanometer scale according to recent research[5-7].Ferric oxides,the focus of this research,have a broad range of applications[8-17],especially in the cancer tumor detection[16],and selective separation and detection of bimolecular due to the apparent simplicity of postsynthesis surface functionalization[17].Stability of the chemical interaction between functional molecules and support is crucial for most medical applications because the support is the key to tracking or targeting treatments that the functional molecule is to perform[18].As a result,it is im-portant to shape-controlled synthesize ferric oxide nanostructures for the enhancement the interaction between functional molecules and ferric oxide nanostructures.
In recent years,a number of methods have been developed to produce ferric oxide nanocrystals possessing enigmatic morphogenesis[19-26].However,in both cases,the shape effects on their applications in surface functionalization have rarely been explored.Herein we report a new approach to the synthesis of novel spindle-like α-Fe2O3nanocrystals.In our synthetic strategy,the morphology and structure can be jointly controlled by tuning the fraction of inorganic salt and organic template(IS-OT)in the extremely low precursor concentration reaction system,and the evaporation-induced self-assembly(EISA)method has been employed to accelerate the reaction and recover the synthesized α-Fe2O3with high yields while preserving favorable shape and structure.The spindle-like morphology of the α-Fe2O3nanocrystals synthesized by IS-OT double control self-assembly exhibits much enhanced the chemical interaction between α-Fe2O3nanocrystals and the surface functionalization agent:dopamine (DA).Moreover,this strategy can be extended to synthesizing other transition metal oxide materials and complex oxides with special nanostructures.
In the soft template methods,surfactants have been used to organize silica into different forms[27-31].However,the transition metal oxide nanomaterials are more difficult to synthesize than silica nanomaterials because the transition metal oxide precursors are more reactive to hydrolysis and condensation compared to the precursors of silica,leading to the formation of undesirable non-uniform structures.Thus,the hydrolytic rate of the precursors is a very important factor to form the transition metal oxide nanomaterials.In this study,the rate of hydrolysis of the ferric oxide precursor can be strictly controlled by adjusting two more important affecting factors.Firstly,from the viewpoint of chemical reaction kinetics,the rate of chemical reaction is directly proportional to the reactant concentration,so the hydrolytic rate of the ferric oxide precursor is confrollable by varying the precursor concentration(in this study,the molar ratio of ferric nitrate to H2O is 1.2∶11103.4).Secondly,appropriate precipitant is also essentially needed for the control of the hydrolytic rate of the ferric oxide precursor.In this case,urea is superior to other conventional precipitants such as sodium hydroxide and aqueous ammonia.The additions of sodium hydroxide and aqueous ammonia in the reaction system only cause the formation of disordered structures.The slowly strengthened alkalescence generated by the hydrolysis of urea rendered the perfect adjustment of hy-drolytic rate of ferric nitrate.
The nucleation has been regulated by inorganic salt and organic template double control self-assembly.Recently,Jia et al.[24,26]found that the shape of nanomaterials can be influenced by the presence of phosphate and sulphate in the hydrothermal method.In our approach,magnesium nitrate has been used to regulate the structure of the synthesized hematite.Besides direct influence,we think that the structures of hematite can be indirectly influenced by magnesium nitrate through regulating the structureofcetyltrimethyl-ammoniumbromide(CTAB)micelles. Obviously,the template micellar structure is one of the main factors influencing the shape of synthesized materials in the soft template methods.On the other hand,the micellar aggregation parameters of cationic quaternary ammonium surfactants,such as translational diffusion coefficient of micelle,hydrodynamic radius,ionization fraction,and aggregation number,are greatly influenced by ionic strength[32].So the presence of magnesium nitrate can influence the structure of CTAB micelles and ultimately influence the structure of the synthesized hematite.
It is worth pointing out that the reaction is very difficult to be carried out in such low ferric nitrate concentration,and conventional techniques(such as centrifugation or filtration)are ineffective at recovering the sample synthesized by such low concentration reaction system.EISA is an efficient pathway to the preparation of nanometer-scale materials[33-40].We had used a modified EISA method in a previous study for synthesizing novel macroporous silica cages with tailoring pore architecture,and found that EISA method can accelerate the reaction and allow successful recovery of materials with high yields while preservingfavorableshapeandstructure[41].SotheEISAmethodwasused to promote the reaction and recover the synthesized products in this study.It is remarkable that two types of ferric oxide singlenanocrystals with different morphologies are synthesized in this study(Scheme 1).The synthesized samples are designated as NFO-1(with magnesium nitrate in the reaction system)and NFO-2(without magnesium nitrate in the reaction system).
1 Experimental
1.1 Chemicals
The following chemicals were purchased and used as received without further purification.Cetyltrimethylammonium bromide (AR),ferric nitrate(Fe(NO3)3·9H2O,AR),urea(AR),magnesium nitrate(AR),and ethanol(AR)were purchased from Sinopharm Chemical Reagent Co.,Ltd.Deionized water was used in all experiments.
1.2 Synthesis
The α-Fe2O3nanocrystals(NFO-1)were synthesized as follows:0.4815 g Fe(NO3)3·9H2O and 0.1486 g Mg(NO3)2were dissolved in deionized water(200 mL)before adding CTAB(1.0983 g)and urea(CO(NH2)2,1.1926 g)under stirring for 10 min to form a clear solution.The reaction mixture was kept static reaction for 24 h at room temperature(23℃).The overall mixture composition(molar ratio)was 1.2 Fe(NO3)3∶1.0 Mg(NO3)2∶3.0 CTAB∶20.0 CO(NH2)2∶11103.4 H2O.The mixture was then allowed to age in the airproofed Teflon flask at 85℃under static condition for 24 h.After aged,the mixture was transferred into a beaker and concentrated at 60℃(EISA).When little liquid was remained,the resulted red solid was washed with ethanol(100 mL×2)to remove the surfactants and collected through centrifugation and dried at room temperature.The remainder surfactants were removed by calcinations at 650℃ for 1 h.NFO-2 was synthesized using the NFO-1 synthesis system,except no addition of magnesium nitrate.

Scheme 1 Schematic illustration of the IS-OT double control self-assembly process of ferric oxide single-nanocrystalsTo employ ferric nitrate as the source of the ferric oxide,the hydrolysis of ferric nitrate with the extremely low concentration was initiated through the hydrolysis of urea. The condensations of nuclei were controlled by the presence of the inorganic salt(magnesium nitrate)and organic template(CTAB).After aging withstaticcondition,the productswererecoveredviaevaporation-induced self-assembly(EISA)method.The resulting synthesis red solid was washed with ethanol to remove the surfactants and collected through centrifugation and dried at room temperature.The materials were then heat treated at high temperatures to promote crystallization.
1.3 Characterization
XRD patterns were obtained with a Bruker D8-advance diffractometer by using Cu Kαradiation(40 kV,40 mA)with a step width of 0.01°(2θ)and an acquisition time of 4 s per step.X-ray photoelectron spectroscopy(XPS)measurement was performed on a PHI5500ESCA analyzer.The main parameters were as follows:Mg Kα,200 W,vacuum~10-7Pa.For Raman measurements,a confocal microprobe Raman instrument(RamLab-010, Jobin Yvon Horiba,France)was used.A 632.8 nm He-Ne laser excitation(0.1 mW)and a 50×long working-distance objective (8 mm)were used in this work.The width of the slit and the size of the pinhole were set as 100 and 1000 μm,respectively.Scanning electron microscopy(SEM)images were obtained on JEOL JSM-6700F electron microscope at 5 kV.The samples were first dispersed in ethanol and then were collected using copper grids covered with carbon films for analysis with gold coating. High-resolution transmission electron microscopy(HRTEM) experiments were performed on a JEOL JEM-3010F electron microscope with an acceleration voltage of 300 kV.
1.4 Electrochemical experiments
Electrochemical experiments were performed on a CHI 660C electrochemical analyzer(CH Instruments,Shanghai Chenhua Instrument Corporation,China)with a conventional three-electrode cell.The working electrode used was glassy carbon electrode(Aida Technology Limited Corp in Tianjin,3 mm in diameter).A platinum wire and a saturated calomel electrode(SCE) were used as the counter electrode and the reference electrode, respectively.Prior to each voltammetric experiment,the dissolved oxygen in solution was removed with purified nitrogen.
2 Results and discussion
It is necessary to confirm the chemical composition of NFO-1 considering that magnesium nitrate is added in the reaction system.The energy-dispersive X-ray(EDX)analysis(Fig.1b)confirms the presence of oxygen and ferric elements and the absence of magnesium element.X-ray photoelectron spectroscopy (XPS)also shows the presence of Fe and O components in the NFO-1(Fig.1a).The results of Fe 2p1/2,Fe 2p3/2,and O 1s electron binding energies are 729.7,716.2,and 535.1 eV,which are very similar to the values recorded in the literature[42-43].During the hydrolysis of urea,a pH favorable for the formation of α-Fe2O3single-crystal structure can be achieved,while magnesium nitrate can not be hydrolyzed in such pH condition.So the magnesium nitrate can be removed during the further treatment.Raman spectroscopy is the important technique that could be effectively used to characterize different types of iron oxides.As shown in Fig.1c,which exhibits five strong resonant peaks at about 222,287,408,494,and 608 cm-1in the range of 200-700 cm-1,the positions of the peaks are in good agreement with the typical frequencies observed from α-Fe2O3[44].The wide-angle powder X-ray diffraction(XRD)is another effective method for confirming the structure of NFO-1.As shown in Fig.1d,several well-resolved diffraction peaks are clearly observed in the range of 20°-70°,which can be indexed as(012),(104),(110),(113), (024),(116),(214),and(300)reflections of α-phase of Fe2O3[45-47]. The analysis results reveal that NFO-1 possesses the crystalline α-phase of Fe2O3.
We have further investigated the morphological structure of NFO-1 by SEM,TEM,and the selected area electron diffraction (SAED).As shown in Fig.2(a,b),NFO-1 possesses not only a very unique spindle-like morphology but also very uniform distribution in size.The average dimensions of these nanoparticles are 439 nm in long axis and 299 nm in short axis,and long/short axis ratios is 1.5(see Supporting Information(available free of charge via the internet at http://www.whxb.pku.edu.cn),Table S1).The information about the crystal can be derived from the highresolutionTEM(HRTEM)images.Fig.2(c-e)shows HRTEM images taken from the areas labeled c-e in a high-magnification TEM image of a separate NFO-1 particle(Fig.2b,the upper left inset,see Supporting Information,Fig.S1);they respectively show the lattice structures at the junction between different protuberances,the tip of the protuberance and the particle.They all clearly show lattice fringes that indicate crystallinity of the entire particle.

Fig.1 XPS(a),EDX(b),Raman(c)spectra,and XRD pattern(d)of NFO-1 after calcination at 650℃
The ideal spindle-like shape of NFO-1 was extremely sensitive to the synthetic conditions,especially the age time and the presence of Mg(NO3)2.In order to understand the formation process of NFO-1,the samples of NFO-11 and NFO-12 were synthesized by varying the crystallization time in the NFO-1 synthesis system(Fig.3).With the prolongation of the crystallization time,the nanprotuberances in the centre of particles grow up to be horn-like outlines,while the highly ordered crystalline walls can be kept(see Supporting Information,Figs.S2 and S3).
Besides the influence of the crystallization time,the morphology and structure of NFO-1 can also be greatly affected by the presence of magnesium nitrate.In order to investigate the effect of magnesium nitrate on the morphology and structure of NFO-1,NFO-2wassynthesizedbasedonthesameprescriptionasNFO-1 except the addition of magnesium nitrate(Fig.4).As shown in Fig.4(a,b),NFO-2 contains spherical or ellipsoidal shape with uniform size(about 35 nm,see Supporting Information,Table S2).Comparing Fig.4 to Fig.1,it is clearly showed that the morphology of the synthesized NFO-2 is different from NFO-1.To obtain the crystal information of NFO-2,5 nanoparticles are randomly chosen from Fig.4b and characterized using HRTEM, and HRTEM images confirm the single-crystallinity of NFO-2 (Fig.4(c-g),and see Supporting Information,Fig.S4),and the XRD pattern reveals that NFO-2 also possesses the crystalline αphase of Fe2O3(Fig.4h).The observation suggests that the morphology and structure of the synthesized samples have been clearly influenced by magnesium nitrate and the mechanism for the formation of NFO-1 may involve the participation of magnesium nitrate.
Under the magnesium nitrate and CTAB double control,there are two possible routes to form NFO-1 nanocrystals(Fig.5). Route I:during the nucleation of Fe2O3species,two types of Fe2O3nuclei had been formed.The small nuclei were adsorbed on the surface of the large nuclei,and the protuberances were formed by the small nucleus.Route II:the protuberances directly grown in the centre of particles.Because of the absence of protuberances on the surface of the tip,Route II may be more possible than Route I.
The method described herein is not limited to the synthesis of single α-Fe2O3nanocrystals,other novel transition metal oxide nanocrystals and complex oxides,such as Mn3O4[48],Co3O4,CuO, and SiO2/Fe2O3,are also applicable in the NFO-2 synthesis system(see Supporting Information,Fig.S5,Fig.S6,and Fig.S7). The results reveal that the self-assembling actions of precursors are controllable in the extremely low precursor concentration re-action system and this method could be extended to synthesize other novel nanomaterials.

Fig.2 SEM and TEM images of NFO-1(a)SEM image;(b)low-magnification TEM image,The inset shows the highmagnification TEM image of a separate NFO-1 particle,scale bar is 100 nm; (c-e)HRTEM images recorded in different regions in the upper inset of b showing the well-defined single nanocrystalline nature

Fig.3 SEM images of(a)NFO-1,(b)NFO-11,(c)NFO-12crystallization time:(a)24 h,(b)48 h,(c)72 h

Fig.4 (a)Low-magnification SEM image,showing the largescale synthesis of NFO-2,(b)low-magnification TEM image of NFO-2,(c-g)HRTEM images recorded in different regions in(b),showing the single crystalline structure,(h)wideangle powder XRD pattern of NFO-2Scale bars in Fig.4(c-g)are 2 nm.
Interestingly,the obtained α-Fe2O3nanocrystals with different morphologies and structures exhibited obviously different surface activities.We choose dopamine(DA)as the surface functional molecule to investigate the chemical interaction between α-Fe2O3nanocrystals and the surface functionalization agent because DA is the important anchor molecule to immobilize the functional molecules on the iron oxide[49].Fig.6a shows the electrochemical responses of DA at the bare and the single α-Fe2O3nanocrystals modified glassy carbon(GC)electrode in phosphate buffer solution at pH 7.0.As shown in Fig.6a,curve GC shows a cyclic voltammogram of the bare glassy carbon electrode at a scan rate of 20 mV·s-1.A weak oxidation peak at 404.3 mV and a weak reduction peak at 18.6 mV can be observed(ΔEp-Bare=385.7 mV),which reveals that there is an electrochemical response of dopamine at the bare GC electrode.But in curve NFO-2,at the NFO-2 modified glassy carbon electrode with the anodic peak potential shifting negatively to 478.6 mV,the corresponding cathodic peak potential is-12.9 mV and ΔEp-NFO-2=491.4 mV.It indicates that the electrochemical redox reaction of dopamine at the NFO-2 modified glassy carbon electrode is more difficult to carry out than that at the bare glassy carbon electrode.However, the cyclic voltammogram of NFO-1 modified glassy carbon electrode(Fig.6a,curve NFO-1)shows a different electrochemical response towards DA from that of the NFO-2 modified glassy carbon electrode.Comparing with the bare and NFO-2 modified glassy carbon electrodes,the remarkable enhancement in the peak currents shows promotional effects of NFO-1.An oxidation peak at 282.9 mV and a reduction peak at 87.1 mV (ΔEp-NFO-1=195.7 mV)are clearly observed.These results reveal that the electron transfer velocity on the surface of NFO-1 is faster than that on the surface of NFO-2 and the better reversibility of the electrode reaction on the surface of NFO-1 than the latter,and a firmer attachment of DA was achieved in the surface of NFO-1.Commonly,the surface effects of nanomaterials will be strengthened with the decrease of particle size.But in this study,NFO-2 with smaller particle size exhibited weaker surface effects than NFO-1.We presume that the special spindlelike morphology of NFO-1 may lead to this result.Because of the presence of nanoprotuberances in the mid section of particles,the appropriate active sites may be available for DA sorption,benefiting to the enhanced chemical interaction between substrate and DA.This hypothesis can be confirmed by the CV experiments of NFO-11 and NFO-12.The CV experiments were carried out under similar experimental conditions,and the results were both almost similar to NFO-2.With the prolongation of the age time,the nanprotuberances of NFO-11 and NFO-12 grew up to be horn-like outlines,and NFO-11 and NFO-12 could not keep the special spindle-like morphology.So the samples could not provide appropriate active sites for DA sorption.
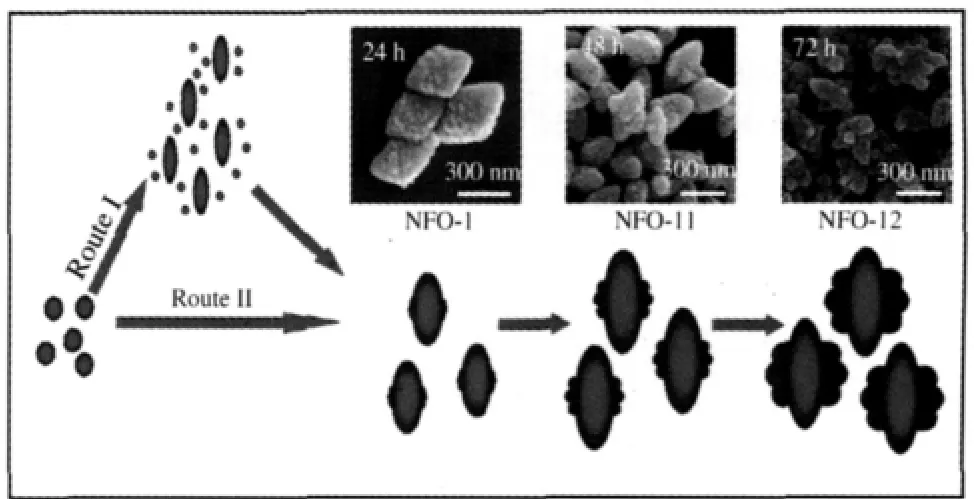
Fig.5 Possible formation and growth process of NFO-1Insets are TEM images from Fig.3.

Fig.6 (a)Cyclic voltammograms obtained for DA on GC electrode,NFO-1 and NFO-2 modified GC electrodes; (b)continuous cyclic voltammograms obtained for DA at NFO-1 modified GC electrodeOne mg NFO(NFO-1 or NFO-2)was ultrasound dispersed in ethanol(2 mL) for 10 min.Then 10 μL of this suspension was coated on GC and dried.The electrochemical responses of DA at the bare and the NFO-1 mesoporous single nanocrystals modified GC were tested in pH 7.0 phosphate buffer solution (1.0×10-4mol DA was added).
The types of interaction between NFO-1 and dopamine have been confirmed by a continuous CV experiment of NFO-1 modified glassy carbon electrode(Fig.6b).Seen from continuous cyclic voltammograms,it is found that the oxidation peak current of the first cycle is higher than that of the second cycle and after the third cycle the peak currents tend to be stable,which reveals that the adsorption process of oxidation state of dopamine controls the process of electrode reaction.While the reduction peak current is almost unchanged after several cycles,this indicates that the diffusion process of reduction state of dopamine controls the process of electrode reaction.
3 Conclusions
In conclusion,the novel α-Fe2O3nanocrystals(NFO-1)with crystalline structures have been synthesized by IS-OT double control self-assembly method in the extremely low concentration system.The obtained α-Fe2O3nanocrystals with different shapes exhibited obviously different surface activities.The capability of surface functionalization of NFO-1 is obviously enhanced because of its special spindle-like morphology.In addition,the synthesis method described herein is also suitable for the synthesis of other transition metal oxide single nanocrystals (such as Mn3O4,Co3O4,and CuO)and complex oxides(Fe2O3/ SiO2).The action mechanism,in which magnesium nitrate or other inorganic salts influence the morphology and structure of transition metal oxide single nanocrystals in the soft template synthesis process,will be an interesting research worth pursuing.
Supporting Information Available:free of charge via the internet at http://www.whxb.pku.edu.cn.
1 Lin,Y.;Wu,S.;Hung,Y.;Chou,Y.;Chang,C.;Lin,M.;Tsai,C.; Mou,C.Chem.Mater.,2006,18:5170
2 Yada,M.;Ohya,M.;Machida,M.;Kijima,T.Langmuir,2000,16: 4752
3 Nelson,P.;Elliott,J.M.;Attard,G.S.;Owen,J.R.Chem.Mater., 2002,14:524
4 Teng,X.;Han,W.;Ku,W.;Hücker,M.Angew.Chem.Int.Edit., 2008,47:2055
5 Srivastava,D.N.;Perkas,N.;Gedanken,A.;Felner,I.J.Phys. Chem.B,2002,106:1878
6 Jiao,F.;Bruce,P.G.Angew.Chem.Int.Edit.,2004,43:5958
7 Jiao,F.;Jumas,J.C.;Womes,M.;Chadwick,A.V.;Harrison,A.; Bruce,P.G.J.Am.Chem.Soc.,2006,128:12905
8 Epling,W.S.;Hoflund,G.B.;Weaver,J.F.;Tsubota,S.;Haruta, M.J.Phys.Chem.,1996,100:9929
9 Pickard,J.M.;Jones,E.G.Energy&Fuels,1997,11:1232
10 Lai,J.;Shafi,K.V.P.M.;Loos,K.;Ulman,A.;Lee,Y.;Vogt,T.; Estournès,C.J.Am.Chem.Soc.,2003,125:11470
11 Wu,C.;Yin,P.;Zhu,X.;Ouyang,C.;Xie,Y.J.Phys.Chem.B, 2006,110:17806
12 Tang,B.;Wang,G.;Zhuo,L.;Ge,J.;Cui,L.Inorg.Chem.,2006, 45:5196
13 Yamada,K.;Mukaihata,N.;Kawahara,T.;Tada,H.Langmuir, 2007,23:8593
14 Zhong,Z.;Ho,J.;Teo,J.;Shen,S.;Gedanken,A.Chem.Mater., 2007,19:4776
15 Han,L.;Shan,Z.;Chen,D.;Yu,X.;Yang,P.;Tu,B.;Zhao,D. J.Colloid Interface Sci.,2008,318:315
16 Kenning,G.G.;Rodriguez,R.;Zotev,V.S.;Moslemi,A.;Wilson, S.;Hawel,L.;Byus,C.;Kovach,J.S.Rev.Sci.Instrum.,2005,76: 014303
17 Perez,J.M.;Simeone,F.J.;Saeki,Y.;Josephson,L.;Weissleder, R.J.Am.Chem.Soc.,2003,125:10192
18 Shultz,M.D.;Reveles,J.U.;Khanna,S.N.;Carpenter,E.E. J.Am.Chem.Soc.,2007,129:2482
19 Rockenberger,J.;Scher,E.C.;Alivisatos,A.P.J.Am.Chem.Soc., 1999,121:11595
20 Woo,K.;Lee,H.J.;Ahn,J.P.;Park,Y.S.Adv.Mater.,2003,15: 1761
21 Wang,X.;Zhuang,J.;Peng,Q.;Li,Y.Nature,2005,437:121
22 Deng,H.;Li,X.;Peng,Q.;Wang,X.;Chen,J.;Li,Y.Angew. Chem.Int.Edit.,2005,44:2782
23 Vayssieres,L.;Sathe,C.;Butorin,S.M.;Shuh,D.K.;Nordgren,J.; Guo,J.Adv.Mater.,2005,17:2320
24 Jia,C.;Sun,L.;Yan,Z.;You,L.;Luo,F.;Han,X.;Pang,Y.; Zhang,Z.;Yan,C.Angew.Chem.Int.Edit.,2005,44:4328
25 Sun,S.;Zeng,H.;Robinson,D.B.;Raoux,S.;Rice,P.M.;Wang, S.;Li,G.J.Am.Chem.Soc.,2004,126:273
26 Jia,C.;Sun,L.;Luo,F.;Han,X.;Heyderman,L.;Yan,Z.;Yan,C.; Zheng,K.;Zhang,Z.;Takano,M.;Hayashi,N.;Eltschka,M.; Kläui,M.;Rüdiger,U.;Kasama,T.;Cervera-Gontard,L.;Dunin-Borkowski,R.E.;Tzvetkov,G.;Raabe,J.J.Am.Chem.Soc., 2008,130:16968
27 Lu,Y.;Fan,H.;Stump,A.;Ward,T.L.;Rieker,T.;Brinker,C.J. Nature,1999,398:223
28 Wu,Y.;Cheng,G.;Katsov,K.;Sides,S.W.;Wang,J.;Tang,J.; Fredrickson,G.H.;Moskovits,M.;Stucky,G.D.Nature Mater., 2004,3:816
29 Che,S.;Liu,Z.;Ohsuna,T.;Sakamoto,K.;Terasaki,O.;Tatsumi, T.Nature,2004,429:281
30 Koganti,V.R.;Dunphy,D.;Gowrishankar,V.;McGehee,M.D.; Li,X.;Wang,J.;Rankin,S.E.Nano Lett.,2006,6:2567
31 Zhang,A.;Zhang,Y.;Xing,N.;Hou,K.;Guo,X.Chem.Mater., 2009,21:4122
32 Bieniecki,A.;Wilk,K.A.;Gapiński,K.J.Phys.Chem.B,1997, 101:871
33 Zhang,Y.;Raman,N.;Bailey,J.K.;Brinker,C.J.;Crooks,R.M. J.Phys.Chem.,1992,96:9098
34 Yang,P.;Zhao,D.;Margolese,D.I.;Chmelka,B.F.;Stucky,G.D. Nature,1998,396:152
35 Brinker,C.J.;Lu,Y.;Sellinger,A.;Fan,H.Adv.Mater.,1999,11: 579
36 Yang,P.;Zhao,D.;Margolese,D.I.;Chmelka,B.F.;Stucky,G.D. Chem.Mater.,1999,11:2813
37 Alberius,P.C.A.;Frindell,K.L.;Hayward,R.C.;Kramer,E.J.; Stucky,G.D.;Chmelka,B.F.Chem.Mater.,2002,14:3284
38 Bartl,M.H.;Puls,S.P.;Tang,J.;Lichtenegger,H.C.;Stucky,G. D.Angew.Chem.,Int.Edit.,2004,43:3037
39 Jiang,X.;Brinker,C.J.J.Am.Chem.Soc.,2006,128:4512
40 Pang,J.;Xiong,S.;Jaeckel,F.;Sun,Z.;Dunphy,D.;Brinker,C.J. J.Am.Chem.Soc.,2008,130:3284
41 Zhan,Y.;Cai,B.;Wang,B.;Huang,X.;Zhang,P.;Li,L.;Wu,Z.; Yin,Z.;Chen,Q.J.Mater.Chem.,2008,18:5967
42 Li,Y.;Ge,X.;Zhang,Z.;Ye,Q.Chem.Mater.,2002,14:1048
43 Brezesinski,T.;Groenewolt,M.;Antonietti,M.;Smarsly,B. Angew.Chem.,Int.Edit.,2006,45:781
44 Li,S.;Zhang,H.;Wu,J.;Ma,X.;Yang,D.Cryst.Growth Des., 2006,6:351
45 Chen,M.;Liu,J.;Sun,S.J.Am.Chem.Soc.,2004,126:1950
46 Cao,M.;Liu,T.;Gao,S.;Sun,G.;Wu,X.;Hu,C.;Wang,Z. Angew.Chem.Int.Edit.,2005,44:4197
47 Cao,H.;Wang,G.;Zhang,L.;Liang,Y.;Zhang,S.;Zhang,X. ChemPhyChem,2006,7:1897
48 Zhang,P.;Zhan,Y.;Cai,B.;Hao,C.;Wang,J.;Liu,C.;Meng,Z.; Yin,Z.;Chen,Q.Nano Res.,2010,3:235
49 Xu,C.;Xu,K.;Gu,H.;Zheng,R.;Liu,H.;Zhang,X.;Guo,Z.;Xu, B.J.Am.Chem.Soc.,2004,126:9938
