Carotenoids Particle Formation by Supercritical Fluid Technologies*
2009-05-12QUANCan全灿JohanCarlforsandCharlottaTurner
QUAN Can (全灿), Johan Carlfors and Charlotta Turner
Carotenoids Particle Formation by Supercritical Fluid Technologies*
QUAN Can (全灿)1, Johan Carlfors2and Charlotta Turner3,**
1Division of Chemistry, National Institute of Metrology, Beijing 100013, China2Department of Pharmaceutics, Faculty of Pharmacy, Uppsala University, Uppsala, SE75123, Sweden3Department of Analytical Chemistry, Uppsala University, Uppsala, SE75124, Sweden
Based on the solubility in supercritical CO2, two strategies in which CO2plays different roles are used to make quercetine and astaxanthin particles by supercritical fluid technologies. The experimental results showed that micronized quercetine particles with mean particle size of 1.0-1.5 µm can be madesolution enhanced dispersion by supercritical fluids (SEDS) process, in which CO2worked as turbulent anti-solvent; while for astaxanthin, micronized particles with mean particle size of 0.3-0.8 µm were also made successfully by rapid expansion supercritical solution (RESS) process.
quercetine, astaxanthin, rapid expansion of supercritical solution, solution enhanced dispersion by supercritical fluids, particle formation
1 Introduction
Carotenoids are organic pigments that are naturally occurring in plants and some other photosyntheticorganisms such as algae, some types of fungus and bacteria. There are over 600 known carotenoids, which are divided into two classes, xanthophylls and carotenes [1-3]. In the past carotenoids were usually isolated from natural sources, such as onions or shrimp shells, by solvent extraction, but the worldwide market is now dominated by synthetic methods, plus some industrial fermentation from fungi and algae cultures as a new way to produce carotenoids such as astaxanthin [4]. In any case, the final product is obtained by crystallization in some conventional processes, either by temperature drop or salting out methods.
The well-known conventional processes for particle size redistribution of solid materials are crushing [5] or grinding [6], which for some compounds is carried out at cryogenic temperatures [7], air micronization, sublimation [8], and recrystallization from solution [9]. There are several practical problems associated with the above-mentioned processes. Some substances are unstable under conventional milling conditions, and in recrystallization processes, the product is contaminated with solvent and waste solvent streams are produced [10].
Application of supercritical fluid technologies may overcome the drawbacks of the conventional processes for formation of solid particles [11-13]. In these processes, supercritical precipitation or crystallization of target substance in a carriersupercritical fluids has become of interest, in which supercritical fluid is used as a dissolution media [14] or as an anti-solvent [15]. Rapid expansion of supercritical solution (RESS) [14, 16, 17] is based on the principle that the solubility can be dramatically reduced by decreasing the solvent density, which depends on the temperature and pressure, resulting in the crystallization of dissolved solutes. The RESS process can be applied to micronize non-polar compounds soluble in supercritical carbon dioxide (SC-CO2). The solution enhanced dispersion by supercritical fluid (SEDS) process is a novel technology developed by Bradford Uni versity [18]. A nozzle with two coaxial passages is used to introduce SC-CO2and a solution of target substances, which are mostly polar, into the particle formation vessel, in which the pressure and temperature are controlled to facilitate the control of particle characteristics. The high velocity of SC-CO2allows the solution to be broken into very small droplets, resulting in the formation of fine particles.
Experimental results are presented in this paper to explore the applicability of these two supercritical fluid techniques for the formation of carotenoids particles and for controlling their characteristics, mainly by studying the processing parameters including pressure and temperature. Based on the solubility of compound in supercritical CO2, two different approaches are applied. Quercetine, the most common polar flavonoid in diet [19], is used as a model substance in SEDS process since it is present in various plants and is a widely studied flavonoid [20]. In the RESS process, astaxanthin is used as it is a “super vitamin E” with antioxidant activity, as high as 100 times more than α-tocopherol [21] and it is a non-polar compound.
2 Experimental
2.1 Materials
Quercetine and astaxanthin with purity of 98% were purchased from Sigma (Steinheim, Germany). Ethyl acetate and-hexane, were all of HPLC grade and used without further purification. Liquid carbon dioxide with purity of 99.9 % was used in both SEDS and RESS process.
2.2 Conventional re-crystallization of quercetine and astaxanthin
Conventional crystallizations of quercetine and astaxanthin were first carried out in ethyl acetate and-hexane solutions respectively, which were as reference processes to compare with the supercritical fluid techniques. Ten milliliters of quercetine saturated ethyl acetate solution and astaxanthin saturated-hexane solution were prepared and then kept at room temperature to evaporate the solvent in darkness under N2. Finally, the crystals were collected for scanning electron microscopy (SEM) particle analysis.
2.3 Preparation of quercetine particle by SEDS technique

The flow rates of CO2and quercetine solution were 10 g·min-1and 0.2 ml·min-1, respectively, at all combinations of pressure and temperature according to the preliminary studies. The quercetine solution of ethyl acetate (1.4 mg·ml-1) was used throughout all the experiments. During the particle formation in SEDS processes, the pressure was varied between 10 and 20 MPa and the temperature ranged from 40 to 60°C.

Figure 1 Schematic representation of (a) the SEDS apparatus and (b) cross section of the coaxial nozzle
Figure 2 Schematic diagram of the RESS apparatus
2.4 Preparation of astaxanthin particle by RESS technique
A schematic diagram of the RESS equipment was given in Fig. 2. A certain amount of astaxanthin powder was first loaded into the 100-ml stainless steel pre-expansion vessel, in which the temperature was controlled by a heating jacket. Liquid carbon dioxide cooled to around 4°C was delivered into the 100-ml vessel at about 6 MPa and first heated to the setup temperature, and then it was compressed to the predetermined pressure (10-30 MPa) by a high pressure pump. The astaxanthin powder was then dissolved in SC-CO2with agitation, and after one hour, the astaxanthin-CO2solution was sprayed through a sapphire nozzle into an expansion chamber at atmospheric pressure. Particles were collected directly on a SEM target substrate inside the chamber. Several drops of water were carefully added onto the particles to prepare the suspended solution sample, which was then ultrasonicated for a certain time to make sure that the particles in the suspension were well separated, after that it was dried gently in darkness with hot N2gas. The specimen was then taken for further characterization.
2.5 Particle characterization
The particle size and morphology of the sample specimen was analyzed by scanning electron microscopy (LEO 1550 EDS/OPAL/EBSD/STEM, Zeiss) after coated with a thin gold/palladium film with the aid of a sputter coater SC7640 (Quorum Technologies, UK).
2.6 Particle size distribution
The particle size distribution was obtained according to the following procedures. First, 200 randomly selected, well-separated particles from the SEM image were measured in zoom-in mode using Matlab, in which individual particles can be recognized clearly, and the longest distance observed across each particle was taken as the particle diameter for simplicity. Secondly, the particle size was calculated based on the ratio of their diameters to the SEM magnification scale in Matlab. And finally, a histogram for particle size distribution was drawn, the mean particle size diameter,(µm), and the standard deviation,(µm), in normal distribution mode(,) were estimated. The measured mean particle size of 240 nm polystyrene obtained from the above method was about 220 nm with relative standard deviation of 8%, indicating that this method was reliable.
3 Results and discussion
3.1 Conventional crystallization
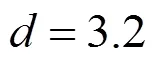

Figure 3 SEM photographs of unprocessed and conventionally recrystallized samples of quercetine from ethyl acetate
For the recrystallization of astaxanthin from-hexane, a typical SEM image for astaxanthin particles is given in Fig. 4, showing flakes-like structure with mean width of 4.8 µm [Fig. 4 (b)] and that from the unprocessed regular crystals with average size of 4.2 µm [Fig. 4 (a)], indicating that the conventional crystallization also leads to a larger particle size. Fig. 4 also indicates that even the morphology of astaxanthin particles is changed before and after the recrystallization.
3.2 SEDS crystallization
The effects of pressure and temperature on the size of quercetine particles were investigated during the SEDS process. In the experiments, all the other variables were kept constant including the solvent flow rate of 0.2 ml·min-1, SC-CO2flow rate of 10.0 g·min-1and the concentration of solution 1.4 g·ml-1.
In Fig. 5, the SEM micrographs show the change in morphologies and sizes of quercetine particles obtained under different experimental conditions. The precipitates obtained under all experimental conditions were needle-like particles or flakes with average particle size around 1-3 µm. Comparing the sizes of micronized particles with that of unprocessed quercetinecrystals, it can be concluded that the SEDS equipment could be used to make micronized quercetine.
SEDS process allows simultaneous dispersion, solvent extraction and particle formation, in which the target solution meets the supercritical carbon dioxide in the coaxial nozzle, producing a supersaturated solute. The turbulent, high-velocity flow promotes both mixing and particle formation. In this study, the particle size from the SEDS (1.0-1.5 µm) is typical 6-9 times smaller than that from the conventional crystallization with size about 9 µm.

Figure 4 SEM photographs of unprocessed and conventionally recrystallized samples of astaxanthin from-hexane
Figure 5 SEM micrographs of quercetine particles collected under different conditions
The effect of operating pressure on the size of particles was tested at 10 and 20 MPa, also shown in Fig. 5. The effect of pressure is not significant by comparing Figs. 5 (a) and 5 (b) with Figs. 5 (c) and 5 (d) respectively.
For the influence of temperature on particle size, at 10 MPa [Figs. 5 (c) and 5 (d)] or 20 MPa [Figs. 5 (a) and 5 (b)], larger quercetine particles can be obtained by increasing the temperature from 40°C to 60°C. The reason may be that the supersaturation ratio, which is the ratio of solute composition to the saturated solubility under equilibrium conditions, is increased since the saturated solubility of solute in supercritical CO2decreases as the CO2density decreases significantly at higher temperature.
The results also show that quercetine micronized particles made by SEDS process is much smaller than those obtained by conventional solvent recrystallization method. The reason may be that at high velocity of SC-CO2the solution is broken into very small droplets in the SEDS process, resulting in the formation of fine particles.
3.3 RESS crystallization
The effects of pre-expansion pressure and pre- expansion temperature on the astaxanthin particles size were also investigated during the RESS process. The astaxanthin-CO2solution was sprayed through a sapphire nozzle into an expansion chamber at atmospheric pressure and the astaxanthin particles were collected directly on a fixed SEM substrate with spraying distance of 50 mm inside this chamber for 10 seconds.
Figure 6 shows the SEM micrographs of astaxanthin particles obtained under different experimental conditions, which all are sphere-like particles with average particle size around 0.5 µm. The comparison of the sizes and morphology of particles with those of unprocessed astaxanthin crystals shows that the RESS process produces quite different morphology.
The RESS process utilizes the high solvating ability of supercritical carbon dioxide (SC-CO2) to make fine (nano to micro-sized) particles [13, 14]. After dissolving the solute in SC-CO2, an extremely fast phase transfer from the supercritical to the gas-like state takes place during the expansion into atmospheric condition. Because of the high supersaturation in SC-CO2, extremely small particles can be formed in the RESS process. In this study, the particle size from the RESS (0.3-0.8 µm) was typical 10 times smaller than that from the conventional crystallization with mean size of 5 µm.
The effect of the pre-expansion pressure on the size of particles was tested at 15 and 30 MP, as reported in Fig. 6. The mean particle size of astaxanthin decreases as the pressure increases from 15 to 30 MPa by comparing Figs. 6 (c) and 6 (d) with Figs. 6 (a) and 6 (b) respectively. The reason may be that increasing pre-expansion pressure decreases the critical nucleus size (the specific size determined by the competition between the aggregate curvature and the free energy favoring the growth of the new phase [13]) and thus produces smaller particles.
The effect of pre-expansion temperature was investigated at 40 and 60°C, at two pre-expansion pressures 20 and 30 MPa. The SEM images with their corresponding particle size distributions are shown in Fig. 6. It indicates that smaller particles are produced at higher pre-expansion temperature, by comparing Figs. 6 (a) and 6 (c) with Figs. 6 (b) and 6 (d), respectively. This might be attributed to that lower temperature results in earlier solute nucleation and higher nuclei concentration during spraying.

Figure 6 SEM micrographs of astaxanthin particles collected under different conditions
Acknowledgements
..
1 Goodwin, T.W., “Nature and distribution of carotenoids”,, 5 (1), 3-13 (1980).
2 Ciapara, H.I., Valenzuela, F.L., Goycoolea, F.M., “Astaxanthin: A review of its chemistry and applications”,, 46 (2), 185-196 (2006).
3 Bouvier, F., Isner, J.C., Dogbo, O., Camara, B., “Oxidative tailoring of carotenoids: A prospect towards novel functions in plants”,, 10 (4), 187-194 (2005).
4 Klingner, A., Hundeshagen, B., Kernebeck, H., “Localization of the yellow pigment formed in roots of gramineous plants colonized by arbuscular fungi”,, 185 (1/2), 50-57 (1995).
5 Kryuchkov, Y.N., “Equipment for fine crushing of ceramic materials (Review)”,, 52 (7/8), 210-215 (1995).
6 Kopac, J., Krajnik, P., “High-performance grinding—A review”,, 175 (1-3), 278-284 (2006).
7 Wilczek, M., Bertling, J., Hintemann, D., “Optimised technologies for cryogenic grinding”,, 74 (S1), S425-S434 (2004).
8 Law, J., Vandijk, D., “Sublimation as a geomorphic process: A review”,, 5 (4), 237-249 (1994).
9 Cisternas, L.A., Vasquez, C.M., Swaney, R.E., “On the design of crystallization-based separation processes: Review and extension”,., 52 (5), 1754-1769 (2006).
10 Knez, Z., Weidner, E., “Particles formation and particle design using supercritical fluids”,, 7 (4/5), 353-361(2003).
11 Shariati, A., Peters, C.J., “Recent developments in particle design using supercritical fluids”,, 7 (4/5), 371-383(2003).
12 Niu, F.H., Subramaniam, B., “Particle fluidization with supercritical carbon dioxide: experiments and theory”,, 46 (10), 3153-3156 (2007).
13 Hakuta, Y., Hayashi, H., Arai, K., “Fine particle formation using supercritical fluids”,, 7 (4/5), 341-351 (2003).
14 Hermsdorf, D., Dana, J., Stephan, S.R., “Formation and stabilization of ibuprofen nanoparticles by pulsed rapid expansion of supercritical solutions”,, 105(8), 951-960 (2007).
15 Reverchon, E., “Supercritical antisolvent precipitation of micro- and nano-particles”,, 15 (1), 1-21 (1999).
16 Ye, X.R., Wai, C.M., “Making nanomaterials in supercritical fluids: A review”,..., 80, 198-204 (2003).
17 Reverchon, E., Adami, R., “Nanomaterials and supercritical fluids”,, 37 (1), 1-22 (2006).
18 Hanna, M., York, P., “Method and device for forming particles”, U.S. Patent, 95/01221 (1994).
19 Hertog, M.G.L., Hollman, P.C.H., Katan, M.B., “Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands”,, 40, 2379-2383 (1992).
20 Erlund, I., “Review of the flavonoids quercetine, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology”,, 24 (10), 851-874(2004).
21 Miki, W., “Biological functions and activities of animal carotenoids”,, 63 (1), 141-146 (1991).
2008-04-14,
2009-02-17.
* Supported partially by the China Ministry of Science and Technology for the China’s Agenda 21 Strategic Research (MOST, 2008IM021900) and the General Administration of Quality Supervision Inspection and Quarantine of the People’s Republic of China for the 4th Food Safety Research (AQSIQ 2008: ASPAQ0809).
** To whom correspondence should be addressed. E-mail: charlotta.turner@kemi.uu.se
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Design and Performance Analysis of Micro Proton Exchange Membrane Fuel Cells*
- Isolation of Cordyceps ophioglossoides L2 from Fruit Body and Optimization of Fermentation Conditions for Its Mycelial Growth*
- Efficient and Comprehensive Utilization of Hemicellulose in the Corn Stover*
- Simulating Surface Aeration Systems at Different Scale of Mixing Time*
- Kinetics of Reaction-Crystallization of Struvite in the Continuous Draft Tube Magma Type Crystallizers—Influence of Different Internal Hydrodynamics
- Preparation and Characterization of Tungsten-substituted Molybdophosphoric Acids and Catalytic Cyclodehydration of 1,4-Butanediol to Tetrahydrofuran*
