Removal of H2S during Anaerobic Bioconversion of Dairy Manure*
2009-05-12JamSaifullahLarandLIXiujin李秀金
Jam Saifullah Larand LI Xiujin (李秀金)
Removal of H2S during Anaerobic Bioconversion of Dairy Manure*
Jam Saifullah Lar1,2and LI Xiujin (李秀金)1,**
1Department of Environmental Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China2Department of Chemistry, Government Degree College, Uch Sharif, Bahawalpur, Pakistan
The main aim of this research was the experimental study at lab scale to check the absorption technology for theremoval of H2S from biogas during anaerobic digestion process. The reagent FeCl3was used to check the removal efficiency of H2S produced from dairy manure during anaerobic bioconversion process. The experiments were conducted under mesophilic conditions. The composition of biogas was analyzed by gas chromatography analyzer equipped with flame photometer and thermal conductivity detectors. Experimental results under the same conditions demonstrate that high concentration of H2S in the form of FeS can be removed totally from the biogas using FeCl3dosing with in anaerobic batch digester.
anaerobic digestion, biogas, dairy manure, FeCl3, hydrogen sulfide
1 INTRODUCTION
Energy is an integral part of society and plays a pivotal role in its socioeconomic development by raising the standard of living and the quality of life. For sustainable development, energy problems are increasing in the world. In recent years, public and political sensitivities to environmental issues and energy security have led to the promotion of renewable energy resources, which are important because they replace fossil fuels. Biomass conversion technology is getting more and more importance because of its huge natural abundance; as per an estimate globally, photosynthesis produces 220 billion ton (dry) of biomass each year with 1% conversion efficiency [1]. The waste management has become a major concern around the world recently.


Table 1 Characteristics of dairy manure and seeding sludge
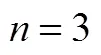
2 MATERIALS AND METHODS
2.1 Feed, seed, and reagent


Table 2 Energy dispersive X-ray (EDX) elemental analysis of dairy manure and seeding sludge
2.2 Apparatus and experimental design
Batch digesters used were Erlenmeyer flasks (2L) with working volume 1.5 L, as they can be operated and maintained simply and conveniently. Butyl rubber stoppers were used to close the mouth of anaerobic digesters and infusion bottles. The junction sites of butyl rubbers and glass were sealed with glue using glue gun in order to avoid leakage of biogas. Natural rubber tubes of 5 mm internal diameter were used for biogas transfer from batch digester to infusion bottles. Digesters were mounted on shakers (model-HZQ-C) made by Herbin Dongming Medical Instrument Factory (China) and were adjusted at 120 r·min-1. The experiments were conducted at (35±1)°C (mesophilic conditions).
In this research, we have employed organic loading rate (OLR) as total solid of dairy manure per unit of the volume of batch digester. Two different OLRs of 60 g·L-1and 80 g·L-1with different concentrations of FeCl3were tested .OLR of 60 g·L-1was tested with FeCl3from 0.1%-12% and OLR of 80 g·L-1was tested with FeCl3from 0.5%-4% and the concentration of FeCl3was used as percentage of TS of dairy manure. The time period for each OLR test was 50-days and each OLR was tested thrice. The operational performance parameter was used to check: (1) The H2S removal efficiency (RE, %), (2) gas quality in terms of gas composition (methane contents, %), (3) biodegradability in terms of daily biogas production & ultimate biogas yield, (4) economical estimation of FeCl3consumption. The operational monitoring parameters were temperature, rotating speed, and pH.
2.3 Analytical methods
Total solids (TS), volatile solids (VS) of dairy manure and total suspended solids (TSS), mixed liquor suspended solids (MLSS) of seeding sludge were measured thrice using standard methods of APHA-1998. Elemental analysis of dairy manure & seeding sludge was done using energy dispersive X-ray (EDX) technique. The physical nature of dairy manure and seeding sludge was observed using scanning electron microscope (SEM). The pH was measured using Thermoelectron pH-meter (model-868). Total ammoniacal nitrogen (TAN) & total Kjeldahl nitrogen (TKN) were analyzed by TKN-analyzer (Foss2003-Sweden). Biogas was analyzedgas chromatography, and for this purpose, BeiFenRuiLi(BFRL)-SP-2100 instrument manufactured by Beijing Analytical Instrument Factory (China) was used. Biogas samples were collected from head spaceISO-9001 & ISO-13485 certified syringes made by Shandong Xinhua Medical Instrument Company. The interval between sample collection and sample injection to GC-analyzer was kept as 30 s (seconds) to avoid the decrease in H2S concentration, as we know that H2S has the tendency to adsorb on the surface of plastic (syringe) layer. For the analysis of CH4contents, the GC-analyzer was equipped with a stainless steel column (2 m×3 mm) with thermal conductivity detector (TCD) adjusted at 150°C. The injection and oven temperature were 150°C and 120°C, respectively. For H2S, flame photometer detector (FPD) adjusted at wavelength of 394 nm (for sulfur) and at 150°C temperature was used. The injection and oven temperatures were 150°C and 100°C, respectively. During the operation of GC analyzer, nitrogen gas of 99.998% purity with flow rate of (30±1)ml·min-1at 0.3 MPa pressure was used as carrier gas. Hydrogen gas and air were used at 0.3 MPa and 0.4 MPa pressure, respectively. Each OLR was analyzed ten times (after every 120 h) for CH4contents and fourteen times (after every 90 h) for H2S concentration. Moreover, each anaerobic digester was analyzed thrice and the average results have been used. Since very close agreements have been found among the results of each OLR, therefore, the overall average results have been mentioned and used in tables and graphs.

H2S removal efficiency (%) is calculated using the following equation:

whereeis concentration of H2S in the experimental digester, andbis concentration of H2S in the blank digester.
3 RESULTS AND DISCUSSION
3.1 Phase 1: Organic loading rate of 60 g·L-1
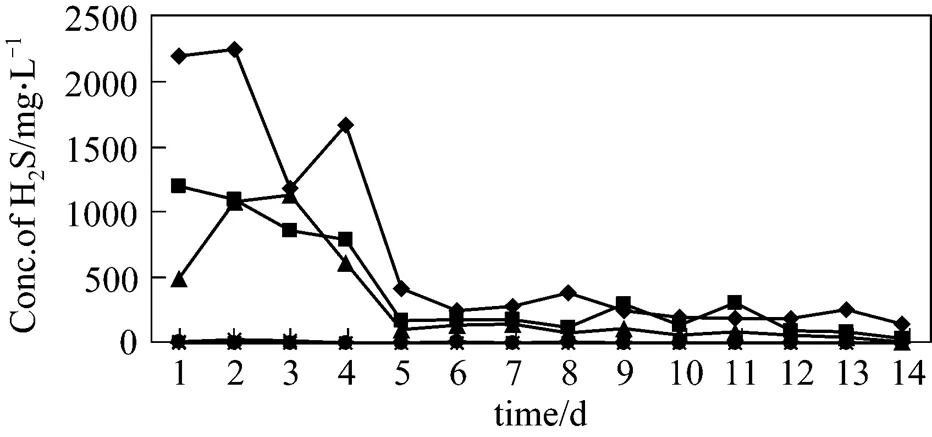
In the first phase, experiments were conducted with organic loading rate (OLR) of 60 g·L-1, using wide range, from 0.1%-12% FeCl3employed to check the removal efficiency of H2S. From Table 3 and Fig. 1, the results demonstrate that from 1%-12% FeCl3digesters, H2S was removed effectively and in most of the batch digesters, the removal efficiency was 100%, while in the previous research mentioned in literature, reduction of H2S concentration in the biogas down to 100 mg·L-1was achieved [15]. The digesters having concentration ranging from 1% to 4% FeCl3produced very little concentration of H2S in the start-up owing to very small amount of reagent, which takes time for thorough mixing. Regarding the biogas production, from Fig. 2 and Table 4, it is clear that ultimate biogas production increases from blank digester to 0.5% FeCl3digester and then it decreases except for 8% FeCl3digester. This exception was owing to the absence of undigested fiber contents as compared to others that were checked by screening the digested slurry by 20 mm screen after the experiments were complete.


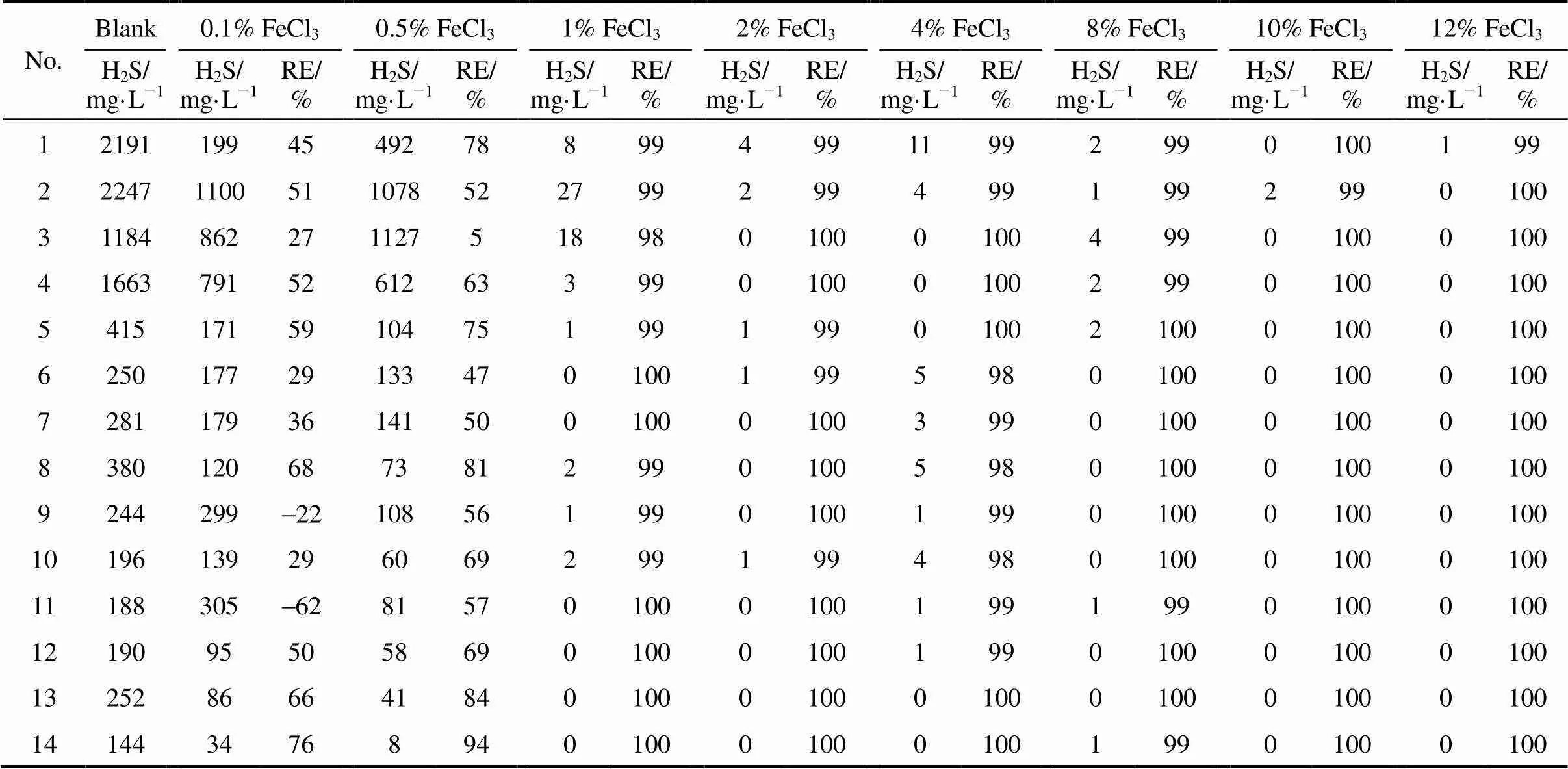
Table 3 Concentration and removal efficiency of H2S with OLR of 60 g·L-1

Table 4 Evaluation of biogas production
3.2 Phase 2: Organic loading rate of 80 g·L-1
In the second phase, experiments were conducted with 80 g·L-1. After getting an idea of removal efficiency from the first phase (OLR 60 g·L-1), we used smaller range (%) of FeCl3. It was observed as it is also clear from Table 5 and Fig. 3 that in 2% FeCl3digester and beyond this concentration, H2S was effectively removed. The pattern of ultimate biogas production shown in Fig. 4 and Table 4 is the same as that observed with OLR 60 g·L-1. The ultimate biogas production increased from blank digester to 1% FeCl3digester and then it decreased up to 4% FeCl3digester. Undigested fiber contents and silicon contents were slightly higher in blank digester and 2% FeCl3digester, which affected the ultimate biogas production.
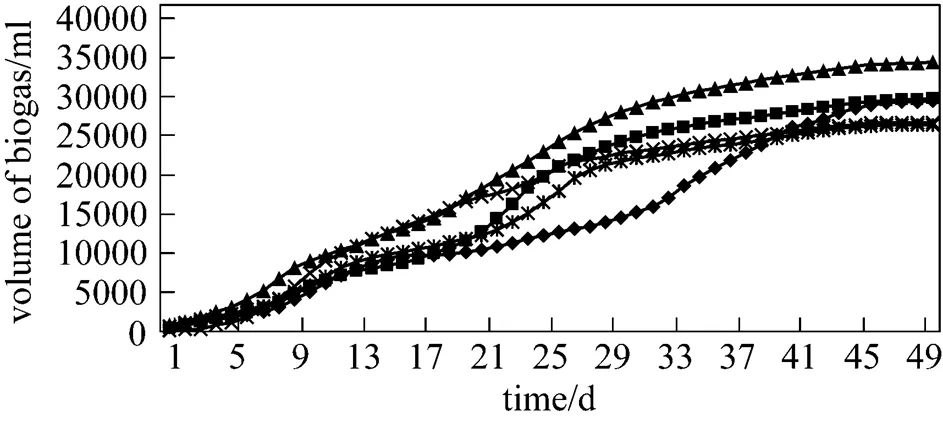


Table 5 Concentration and removal efficiency of H2S with OLR of 80 g·L-1
3.3 Evaluation of the experiment
This process of sweetening of biogas has proved to be very suitable and economically viable as we achieved 100% H2S removal efficiency using small quantity of FeCl3as compared to caustic scrubbers that are expensive and are used in large quantities. FeCl3also proved very effective because of its solubility in aqueous medium (0.92 g·ml-1at 20°C) and FeCl3retards dissimilatory sulfate reduction owing to its Lewis acid (electron deficient) nature because during metabolism, it also accepts electrons along with sulfate ion. This technique is also suitable because of pronounced positive effect of iron on methane production (methanogenesis).
Iron content itself is not toxic to methanogens but in high concentration, it affects indirectly as it forms Fe (OH)3by hydrolysis in this medium, which results in increase of PH values, and consequently, affects the biogas production. This is the reason why with OLR of 60 g·L-1beyond 0.5% FeCl3digester and with OLR of 80 g·L-1beyond 1% FeCl3digester, the ultimate biogas production decreases. The other reason for low ultimate biogas production in both organic loading rates at high concentration of FeCl3is its tendency to form foam with dairy manure, which decreases VS reduction. It was also observed that in the beginning of the process, there was a difference in time among batch digesters for biogas production initiation and was noted that the higher the concentration of FeCl3, the later it started gas production, and even 12% FeCl3digester started biogas production after 8-10 days. The reason is that FeCl3is coagulating agent making biomass semi solid, therefore it takes time for thorough shaking of dairy manure and seeding sludge. The results demonstrate that the concentration of H2S is very high in the beginning and then it decreases showing that Sulfur containing compounds are consumed quickly in the beginning of the process. The resultant product as a result of this chemical reaction, FeS, precipitates in the digester preventing stripping of H2S to biogas and also offers protection against oxygen contamination, which is the basic requirement of this anaerobic bioconversion technology.

FeS being (solubility: 5×10-5mg·L-1at 18°C) insoluble, is non toxic to anaerobic digestion process and plays an important role for the effective removal of H2S.

4 CONCLUSIONS
A chemical absorption process for the removal of H2S has been tested at lab scale with organic loading rates 60 g·L-1and 80 g·L-1. Both calculations and experimental results obtained in this study show that it is possible to achieve the complete removal of H2S from biogas using FeCl3with in anaerobic batch digester that was operated under mesophilic (35±1)°C conditions. This process is very economical as it uses very small quantity (TS of dairy manure) of cheap and common reagent FeCl3. The results presented show that the H2S removal is not only the advantage of this chemical absorption technique but it also enhances the biogas production in terms of ultimate biogas production and methane contents.
ACKNOWLEDGEMENTS
,,,,.
1 Mirza, U.K., Ahmad, N., Majeed, T., “An overview of biomass energy utilization in Pakistan”,..., 12 (7), 1988-1996 (2008).
2 Lastella, G., Testa, C., Cornacchia, G., Notornicola, M., Voltasio, F., Sharma, V.K., “Anaerobic digestion of semi-solid organic waste: Biogas production and its purification”,.., 43, 63-75 (2002).
3 Liao, W., Liu, Y., Liu, C., Wen, Z., Chen, S., “Acid hydrolysis of fibers from dairy manure”,.., 97, 1687-1695(2006).
4 Zicari, S.M., “Removal of H2S by using cow-manure compost”, M.S. Thesis, Cornell Univ., United State of America (2003).
5 Chou, S., Mike, F., Sam, K., Lisa, I., Lara, C., “Toxicological profile for H2S”, U.S. Public Health Services Agency for Toxic Substances and Disease Registry, Atlanta (2006).
6 Maat, H.T., Hogendoorn, J.A., Versteeg, G.F., “The removal of hydrogen sulfide from gas streams using an aqueous metal sulfate absorbent, Part I. The absorption of hydrogen sulfide in metal sulfate solutions”,..., 43, 183-197 (2005).
7 Strevett, K.A., Vieth, R.F., Grasso, D., “Chemo-autotrophic biogas purification for methane enrichment: Mechanism and kinetics”,..., 58, 71-79 (1995).
8 Maat, H.T., Hogendoorn, J.A., Versteeg, G.F., “The removal of hydrogen sulfide from gas streams using an aqueous metal sulfate absorbent, Part II. The regeneration of copper sulfide to copper oxide—An experimental study”,..., 43, 199-213 (2005).
9 Truong, L.V.A., Abatzoglou, N., “A H2S reactive adsorption process for the purification of biogas prior to its use as bioenergy vector”,, 29, 142-151 (2005).
10 Lusk, P., Methane Recovery from Animal Manures: The Current Opportunities Case Book, National Renewable Energy Laboratory, Washington DC (1998).
11 Horikawa, M.S., Rossi, F., Gimenes, M.L., Costa, C.M.M., da Silva, M.G.C., “Chemical absorption of H2S for biogas purification”,..., 21 (3), 415-422 (2004).
12 Hagen, M., Polman, E., “Adding gas from biomass to the gas grid”, Contract No: XVII/4.1030/z/99-412, Final report submitted to Danish Gas Agency (2001).
13 Donald, L., Wise, C., Analysis of System for Purification of Fuel Gas. Fuel Gas Production from Biomass, Vol. 2, CRC Press, Boca Raton, Florida (1981).
14 Kapdi, S.S., Vijay, V.K., Rajesh, S.K., Parsad, R., “Biogas scrubbing, compression and storage: perspective and prospectus in Indian context”,, 30 (8), 1195-1202 (2005).
15 Schomaker, A., “Anaerobic digestion of agro-industrial wastes: Information networks technical summary on gas treatment”, Report of Anaerobic Digestion Network (AD-NETT) Project FAIR-CT96-2083 (DG12-SSMI), Canada (2000).
2008-06-18,
2008-09-26.
the Natural Science Foundation of Beijing (8062023).
** To whom correspondence should be addressed. E-mail: xjli@mail.buct.edu.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Design and Performance Analysis of Micro Proton Exchange Membrane Fuel Cells*
- Isolation of Cordyceps ophioglossoides L2 from Fruit Body and Optimization of Fermentation Conditions for Its Mycelial Growth*
- Efficient and Comprehensive Utilization of Hemicellulose in the Corn Stover*
- Simulating Surface Aeration Systems at Different Scale of Mixing Time*
- Kinetics of Reaction-Crystallization of Struvite in the Continuous Draft Tube Magma Type Crystallizers—Influence of Different Internal Hydrodynamics
- Preparation and Characterization of Tungsten-substituted Molybdophosphoric Acids and Catalytic Cyclodehydration of 1,4-Butanediol to Tetrahydrofuran*
