Copper(II)-mediated cascade cyanomethylation of arylacrylamides to access cyano substituted quinoline-2,4-diones
2022-11-02LILiLIANGuoLUMingkunSUNHuanLIUJikai
LI Li,LIAN Guo,LU Mingkun,SUN Huan,LIU Jikai
(School of Pharmaceutical Sciences,South-Central Minzu University,Wuhan 430074,China)
Abstract A practical and efficient Cu-mediated serial radical addition/cyclization reaction of o-cyanoaryl acrylamide with acetonitrile was described.A copper-mediated cascade cyanomethylation of o-cyanoaryl acrylamide with acetonitrile as the radical precursor to access cyano substituted quinoline-2,4-dione was realized.The cyanomethylated quinoline-2,4-diketone products substituted by various substituents could be obtained with high yield under mild conditions,which provided a new method for introducing cyanomethyl group into various quinoline-2,4-diketones products.The method had the advantages of simple operation,mild reaction condition,wide substrate tolerance and great developmental prospect.
Keywords copper-mediated;radical addition/cyclization;cyanomethylation;quinoline-2,4-dione
Difunctionalization of activated alkenes has proven to be an efficient and powerful synthetic strategy to obtain a range of significant bioactive compounds and intermediates[1-3].Recently,this strategy was applied to the synthesis of functionalized quinoline-2,4-diones which are widely found in natural products and pharmaceuticals with excellent antiplatelet[4],antibacterial[5],and herbicidal activities[6].Oxidative radical addition/cyclization cascade reaction ofo-cyanoarylacrylamides is one of the most general and efficient routes to achieve quinoline-2,4-diones(Fig.1)[7-9].Diverse functional groups,including but not limited to alkyl[10],fluorocontaining group[11-12],nitro group[13],sulfonyl[14],and carbonyl[15],could be incorporated into quinoline-2,4-dione skeletons via using different radical precursors.

Fig.1 Radical addition/cyclization cascade reaction of o-cyanoaryl acrylamides for synthesis of functional quinoline-2,4-diones图1邻氰基芳基丙烯酰胺的自由基加成/环化级联反应合成官能团化的喹啉-2,4-二酮
Recently,the methodologies of introducing difluoromethyl group[16]and methyl group[17]into quinoline-2,4-diones via oxidative difunctionalization ofo-cyanoarylacrylamides under visible light irradiation were reported(Fig.2(a)).Direct cyanomethylation is a valuable transformation since the conversion of cyano group into amino,ester,carboxyl,alkyl,aldehyde,and tetrazole is frequently used in organic synthesis and drug synthesis[18-20].Therefore,the development of practical reagents and efficient methodologies for cyanomethylation are of great significance[21].Direct activation of acetonitrile has been demonstrated as one of the most attractive approaches to introduce cyanomethyl group into organic skeletons due to the highly efficient atom economy[22-24].The copper-catalyzed cyanide reaction is efficient and fast,which has attracted wide attention from organic chemists.Classical strategy to achieve C―H activation of acetonitrile always requires stoichiometric amounts of transition metals such as Rh,Ru,Fe,Ir,Ni,etc.,to form activated Ln-M-CH2CN complexes[25-27].Recently,C―H activation of acetonitrile through oxidative radical pathway has been reported[28].Cyanomethyl group could be incorporated into oxindole skeleton via radical addition/cyclization ofN-arylacrylamides with acetonitrile(Fig.2(b))[29-31].
Based on our continuous interest in oxidative difunctionalization of arylacrylamides and synthetic importance of cyanomethyl group and quinoline-2,4-diones in organic chemistry and medicinal chemistry,this paper herein would like to present a practical and efficient oxidative radical addition/cyclization reaction ofocyanoarylacrylamide with acetonitrile for the construction of cyanomethylated quinoline-2,4-diones(Fig.2(c)).
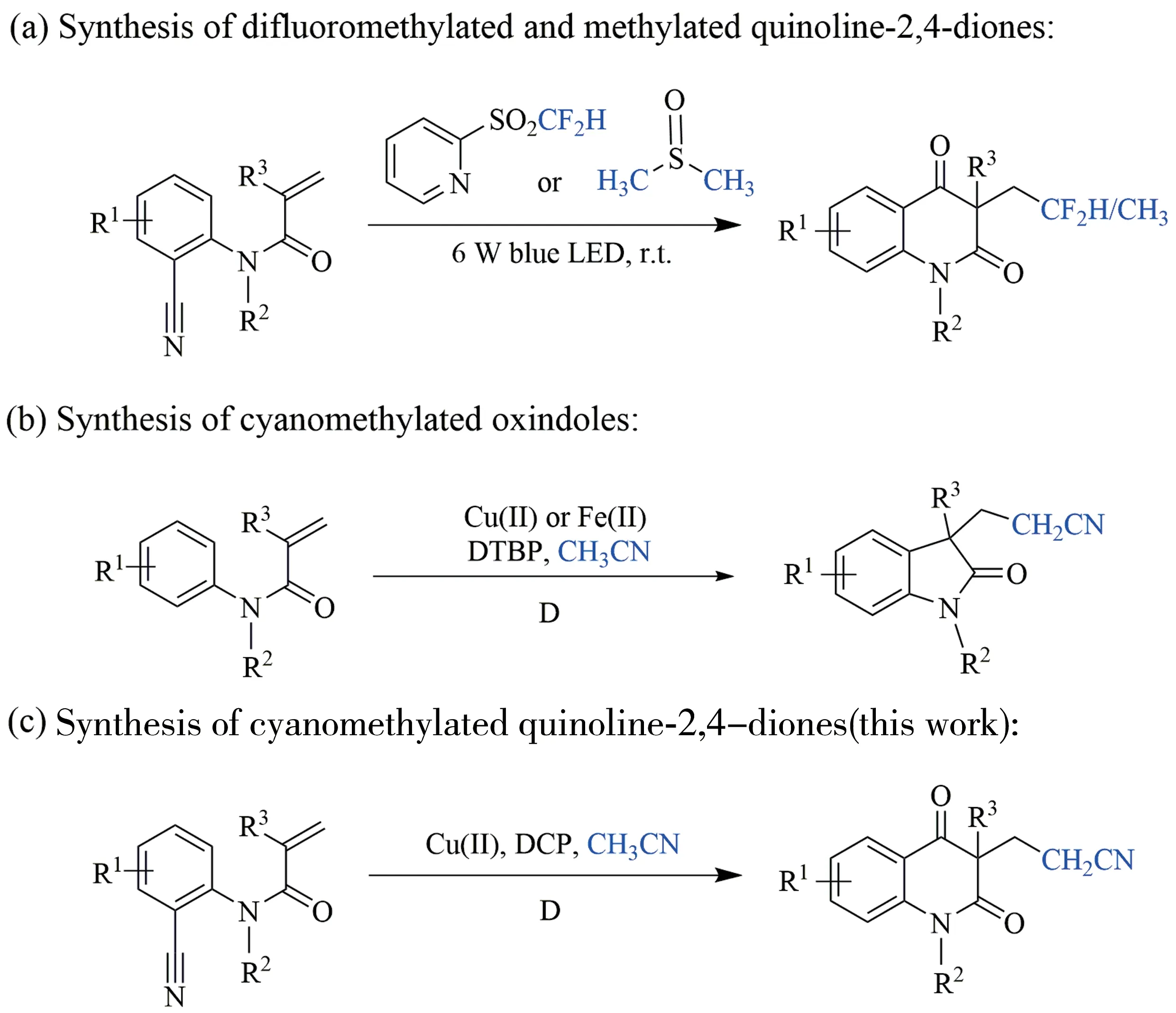
Fig.2 Radical oxidative difunctionalization of arylacrylamides to access substituted oxindoles and quinoline-2,4-diones图2芳基丙烯酰胺的自由基氧化双官能合成氧化吲哚和喹啉-2,4-二酮
1 Experimental
1.1 Reagents and apparatus
N-(2-cyanophenyl)-N-methyl-methacrylamide 1a was synthesized from 2-aminobenzonitrile(Adamas)and methacrylamide(Macklin);Cu(ClO4)2·6H2O(Adamas),dicumyl peroxide(DCP,Adamas),malononitrile,phenylacetonitrile,NaOAc and CH3CN were purchased and used directly;analytical thinlayer chromatography was performed with 0.25 mm coated commercial silica gel plates GF254(60-F250,0.2,Marine Chemical Inc.,Qingdao,P.R.China)using petroleum ether/ethyl acetate;flash column chromatography was performed over silica gel(200-300 mesh ASTM,Marine Chemical Inc.,Qingdao,P.R.China).
1H NMR and13C NMR spectra were collected on Bruker advance-600(1H:600 MHz,CDCl3at 7.26;13C:150 MHz,CDCl3at 77.0);HRMS spctra were performed in Analysis and Test Center,School of Pharmaceutical Sciences,South-Central Minzu University.
1.2 Synthesis of 3-(1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydroquinolin-3-yl)propanenitrile
N-(2-cyanophenyl)-N-methyl-methacrylamide 1a(0.1 mmol),CuCl2(0.05 mmol,molar ratio 50%),DCP(0.3 mmol,3.0 equiv.),NEt3(0.5 equiv.),and TBAF(2.0 equiv.)were weighed in CH3CN/H2O(1 mL,V(CH3CN)∶V(H2O)=3∶1);the mixture was heated at 130℃for 12 h.After the reaction completed,the rotary distillation and concentration process,separation by column chromatography(V(petroleum ether)∶V(ethyl acetate)=10∶1)gave the title compound 3-(1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydroquinolin-3-yl)propanenitrile(2a)as yellow solid.
2 Results and discussion
2.1 Screening reaction conditions
A reaction involvingN-(2-cyanophenyl)-Nmethyl-methacrylamide 1a as the starting material with acetonitrile in the presence of radical initiators(Tab.1)was initialized.The cascade reaction proceeded smoothly and afforded the desired cyanomethylated product 2a in 16% yield with 3.0 equiv of DCP,along with methylated byproduct.Other oxidants were screened and DCP was found more satisfactory than BPO and TBPB.Additionally,a variety of transition metals including Fe(II)salts,Cu(II)salts,and Cu(I)salts were attempted and Cu(ClO4)2demonstrated the highest activity.Further,the influence of the phase transfer catalyst,the base,the ratio of acetonitrile and water were evaluated.Phase transfer catalyst proved to be not necessary for the transformation.Exploration of organic bases and inorganic bases suggested NaOAc(1.0 equiv.)was the best satisfactory candidate.After several permutations and combinations,the optimized conditions were found as follows:Cu(ClO4)2(molar ratio 50%),DCP(3.0 equiv.),NaOAc(1.0 equiv.),CH3CN/H2O(V(CH3CN)∶V(H2O)=3∶1),and 130℃.Tab.1 Screening reaction conditions for cyanomethylation ofo-cyanoarylacrylamide

表1 邻氰基芳基丙烯酰胺氰甲基化的筛选反应条件
2.2 Substrate scope
With the optimal conditions in hand,the scope of functional group tolerance was investigated.As shown in Fig.3,a variety ofo-cyanoarylacrylamide derivatives were smoothly converted to the desired cyanomethylated quinoline-2,4-diones with moderate yields.The protecting groups at the nitrogen were necessary and made great influence on the process and only electrondonating groups including methyl(2a)and benzyl(2b).The substrates with both electron donating groups(i.e.,methoxy,methyl)and electron withdrawing groups(i.e.,chloride,fluoride,bromide,and trifluoromethyl)on the phenyl ring afforded the desired cyanomethylated quinoline-2,4-diones in satisfactory yields.

Fig.3 Substrate scope for cyanomethylation of o-cyanoarylacrylamide图3邻氰基芳基丙烯酰胺氰甲基化的底物范围
Cu(ClO4)2·6H2O(0.05 mol,50%),N-(2-cyanophenyl)-N-methyl-methacrylamide 1(0.1 mol,1.0 equiv.),DCP(0.3 mmol,3.0 equiv.),and NaOAc(0.1 mmol,1.0 equiv.)were sequentially weighed into a sealed tube.CH3CN(0.75 mL)and H2O(0.25 mL)were added with a syringe.The mixture was heated to 130℃stirring for 12 h.The solvent were removed by rotary evaporation and the residue was purified by silica gel flash chromatography using petroleum ether/ethyl acetate to afford cyanomethylated compounds 2a-2t as yellow solid.
3-(1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydroquinolin-3-yl)propanenitrile(2a):57%yield.1H NMR(600 MHz,Chloroform-d)δ8.02(dd,J=7.7,1.6 Hz,1H),7.67(ddd,J=8.4,7.3,1.7 Hz,1H),7.23-7.17(m,2H),3.49(s,3H),2.47-2.41(m,2H),2.32-2.30(m,2H),1.48(s,3H).13C NMR(150 MHz,Chloroform-d)δ195.85,172.11,142.89,136.52,128.32,123.50,119.79,118.87,114.99,56.50,30.69,29.97,26.12,13.42.HRMS(ESI):m/z[M+H]+calcd.for C14H15N2O2243.11335,found 243.11279.
3-(1-benzyl-3-methyl-2,4-dioxo-1,2,3,4-tetrahydroquinolin-3-yl)propanenitrile(2b):33% yield.1H NMR(600 MHz,Chloroform-d)δ8.02(dd,J=7.7,1.6 Hz,1H),7.51(ddd,J=8.8,7.4,1.7 Hz,1H),7.35(t,J=7.5 Hz,2H),7.29(d,J=7.3 Hz,1H),7.22(d,J=7.3 Hz,2H),7.17(t,J=7.5 Hz,1H),7.07(d,J=8.4 Hz,1H),5.51(d,J=16.2 Hz,1H),5.08(d,J=16.1 Hz,1H),2.56-2.47(m,2H),2.40-2.35(m,2H),1.58(s,3H).13C NMR(150 MHz,Chloroform-d)δ195.67,172.48,142.19,136.41,135.71,129.07,128.40,127.63,126.17,123.63,119.99,118.93,115.90,56.71,46.40,30.35,26.42,13.46.HRMS(ESI):m/z[M+H]+calcd.for C20H19N2O2319.14465,found 319.14420.
3-(7-methoxy-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydroquinolin-3-yl)propanenitrile(2d):50% yield.1H NMR(600 MHz,Chloroform-d)δ8.03-7.98(m,1H),6.73(dd,J=8.7,2.2 Hz,1H),6.62(d,J=2.1 Hz,1H),3.92(s,3H),3.46(s,3H),2.45-2.39(m,2H),2.30-2.24(m,2H),1.46(s,3H).13C NMR(150 MHz,Chloroform-d)δ194.02,172.62,166.15,144.79,130.71,118.72,113.43,108.47,100.90,55.88,46.92,30.95,29.74,26.20,13.28.HRMS(ESI):m/z[M+H]+calcd.for C15H17N2O3273.12392,found 273.12311.
3-(6-bromo-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydroquinolin-3-yl)propanenitrile(2e):55%yield.1H NMR(600 MHz,Chloroform-d)δ8.11(d,J=2.3 Hz,1H),7.74(dd,J=8.8,2.4 Hz,1H),7.08(d,J=8.8 Hz,1H),3.47(s,3H),2.45-2.42(m,2H),2.33-2.30(m,2H),1.47(s,3H).13C NMR(150 MHz,Chloroform-d)δ193.72,170.78,140.86,137.98,129.78,120.05,117.71,115.92,115.57,55.59,29.46,29.12,25.17,12.38.HRMS(ESI):m/z[M+H]+calcd.for C14H14BrN2O2321.02387,found 321.02338.
3-(1,3-dimethyl-2,4-dioxo-6-(trifluoromethyl)-1,2,3,4-etrahydroquinolin-3-yl)propanenitrile(2f):48% yield.1H NMR(600 MHz,Chloroform-d)δ8.29(s,1H),7.90(d,J=8.6 Hz,1H),7.31(d,J=8.7 Hz,1H),3.53(s,3H),2.51-2.45(m,2H),2.37-2.32(m,2H),1.50(s,3H).13C NMR(150 MHz,Chloroform-d)δ194.69,172.08,145.27,132.88(q,J=3.4 Hz),125.87(q,J=3.8 Hz),124.44,122.28,119.57,118.62,115.61,56.79,30.33,30.27,26.22,13.37.HRMS(ESI):m/z[M+Na]+calcd.for C15H13F3N2O2Na 333.08268,found 333.08218.
3-(7-fluoro-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydroquinolin-3-yl)propanenitrile(2g):39%yield.1H NMR(600 MHz,Chloroform-d)δ8.06(dd,J=8.6,6.5 Hz,1H),6.93-6.86(m,2H),3.46(s,3H),2.46-2.40(m,2H),2.32-2.29(m,2H),1.48(s,3H).13C NMR(150 MHz,Chloroform-d)δ193.82,171.87,167.31(d,J=257.1 Hz),144.81(d,J=11.8 Hz),130.92(d,J=11.1 Hz),118.26,115.88(d,J=2.5 Hz),110.51(d,J=22.4 Hz),102.23(d,J=27.5 Hz),55.91,30.22,29.68,25.86,12.96.HRMS(ESI):m/z[M+H]+calcd.for C14H14FN2O2261.10393,found 261.10339.
3-(6-fluoro-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydroquinolin-3-yl)propanenitrile(2h):56%yield.1H NMR(600 MHz,Chloroform-d)δ7.69(dd,J=7.9,3.1 Hz,1H),7.38(ddd,J=9.2,7.4,3.1 Hz,1H),7.18(dd,J=9.1,4.0 Hz,1H),3.49(s,3H),2.46-2.43(m,2H),2.34-2.30(m,2H),1.48(s,3H).13C NMR(150 MHz,Chloroform-d)δ194.64,171.21,158.16(d,J=246.3 Hz),138.87(d,J=2.3 Hz),123.07(d,J=23.4 Hz),120.49(d,J=6.4 Hz),118.28,116.43(d,J=7.3 Hz),113.63(d,J=23.4 Hz),55.94,30.10,29.79,25.73,12.95.HRMS(ESI):m/z[M+H]+calcd.for C14H14FN2O2261.10393,found 261.10333.
3-(5-fluoro-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydroquinolin-3-yl)propanenitrile(2i):29% yield.1H NMR(600 MHz,Chloroform-d)δ7.61-7.56(m,1H),6.99(d,J=8.5 Hz,1H),6.93-6.89(m,1H),3.48(s,3H),2.42-2.39(m,2H),2.37-2.32(m,2H),1.48(s,3H).13C NMR(150 MHz,Chloroform-d)δ192.65,170.98,161.69(d,J=266.5 Hz),143.38(d,J=3.4 Hz),136.08(d,J=11.8 Hz),118.47,111.23(d,J=21.4 Hz),110.33(d,J=3.6 Hz),109.32(d,J=10.1 Hz),56.84,30.31,29.51,24.95,12.81.HRMS(ESI):m/z[M+Na]+calcd.for C14H13FN2O2Na 283.08588,found 283.08487.
3-(6-chloro-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydroquinolin-3-yl)propanenitrile(2j):47%yield.1H NMR(600 MHz,Chloroform-d)δ 7.97(d,J=2.5 Hz,1H),7.61(dd,J=8.8,2.5 Hz,1H),7.15(d,J=8.8 Hz,1H),3.48(s,3H),2.47-2.41(m,2H),2.34-2.30(m,2H),1.48(s,3H).13C NMR(150 MHz,Chloroform-d)δ 194.84,171.79,141.43,136.13,129.40,127.81,120.79,118.73,116.65,56.59,30.51,30.18,26.18,13.41.HRMS(ESI):m/z[M+H]+calcd.for C14H14ClN2O2277.07438,found 277.07388.
3-(1,3-imethyl-2,4-dioxo-7-(trifluoromethyl)-1,2,3,4-tetrahydroquinolin-3-yl)propanenitrile(2k):38% yield.1H NMR(600 MHz,Chloroform-d)δ 8.14(d,J=8.0 Hz,1H),7.46(dd,J=8.2,1.5 Hz,1H),7.41(s,1H),3.54(s,3H),2.48-2.45(m,2H),2.37(s,3H),2.36-2.33(m,2H),1.49(s,3H).13C NMR(150 MHz,Chloroform-d)δ 194.41,171.43,147.11,141.96,127.35,123.52,117.88,116.59,114.44,55.24,29.86,28.88,25.19,21.44,12.41.HRMS(ESI):m/z[M+H]+calcd.for C15H17N2O2257.12900,found 257.12836.
3-(1,3,6-trimethyl-2,4-dioxo-1,2,3,4-tetrahydroquinolin-3-yl)propanenitrile(2l):33%yield.1H NMR(600 MHz,Chloroform-d)δ 7.81(d,J=1.8 Hz,1H),7.47(dd,J=8.6,2.0 Hz,1H),7.08(d,J=8.4 Hz,1H),3.47(s,3H),2.44-2.40(m,2H),2.37(s,3H),2.31-2.28(m,2H),1.46(s,3H).13C NMR(150 MHz,Chloroform-d)δ 195.09,170.98,139.72,136.31,132.33,127.20,118.61,117.91,113.98,55.40,29.74,28.92,25.10,19.32,12.42.HRMS(ESI):m/z[M+H]+calcd.for C15H17N2O2257.12900,found 257.12833.
3-(1,3-dimethyl-2,4-dioxo-7-(trifluoromethyl)-1,2,3,4-etrahydroquinolin-3-yl)propanenitrile(2m):38%yield.1H NMR(600 MHz,Chloroform-d)δ 8.14(d,J=8.0 Hz,1H),7.46(dd,J=8.2,1.5 Hz,1H),7.41(s,1H),3.54(s,3H),2.48-2.45(m,2H),2.36-2.33(m,2H),1.49(s,3H).13C NMR(150 MHz,Chloroform-d)δ 194.05,170.80,142.22,136.56(q,J=32.9 Hz),128.28,123.12,120.84,118.97(q,J=3.7 Hz),117.66,55.88,29.28,29.20,28.68,25.12,12.38.HRMS(ESI):m/z[M+H]+calcd.for C15H14F3N2O2311.10074,found 333.10019.
[3-(2-cyanoethyl)-1-methyl-2,4-dioxo-1,2,3,4-etrahydroquinolin-3-yl]methyl acetate(2o):36% yield.1H NMR(600 MHz,Chloroform-d)δ 8.05-8.00(m,1H),7.72-7.67(m,1H),7.25-7.21(m,2H),4.48(d,J=10.2 Hz,1H),4.39(d,J=10.2 Hz,1H),3.52(s,3H),2.40(m,2H),2.31(m,2H),1.84(s,3H).13C NMR(150 MHz,Chloroform-d)δ 192.58,168.80,168.50,142.18,136.09,127.20,122.73,119.57,117.16,114.20,67.55,58.57,30.92,28.69,21.69,19.37.HRMS(ESI):m/z[M+H]+calcd.for C16H17N2O4301.11883,found 301.11819.
3-(6,7-dimethoxy-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydroquinolin-3-yl)propanenitrile(2s):32%yield.1H NMR(600 MHz,Chloroform-d)δ 7.48(s,1H),6.60(s,1H),4.01(s,3H),3.93(s,3H),3.48(s,3H),2.05-1.95(m,2H),1.61(s,2H),1.45(s,3H).13C NMR(150 MHz,Chloroform-d)δ 195.83,173.89,155.10,144.69,139.03,112.58,108.54,108.25,97.52,56.69,55.84,55.75,32.69,29.23,22.76,9.12.HRMS(ESI):m/z[M+H]+calcd.for C16H19N2O4303.13448,found 303.13385.
3-(7-chloro-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydroquinolin-3-yl)propanenitrile(2t):26%yield.1H NMR(600 MHz,Chloroform-d)δ 7.90(d,J=8.8 Hz,1H),7.12(d,J=6.7 Hz,2H),3.41(s,3H),2.39-2.36(m,2H),2.26-2.22(m,2H),1.41(s,3H).13C NMR(150 MHz,Chloroform-d)δ 194.70,172.17,143.86,142.96,129.74,123.80,118.70,118.10,115.35,56.53,30.59,29.69,26.26,13.41.HRMS(ESI):m/z[M+H]+calcd.for C14H14ClN2O2277.07383,found 277.07393.
2.3 Malononitrile and phenylacetonitrile
The evaluation of the steric hindrance effect on the aromatic ring suggested that the substituents at the ortho-position of the nitrile group greatly interferenced the reaction process(2i).Then the substitution effect on the olefin part was tested.The mono-substituted alkene(R3=H)was ineffective in this reaction and failed to give the desired product.Thegem-disubstituted substrates(R3=acetoxymethyl)smoothly furnished the corresponding quinoline-2,4-diones in 36% yield.To further evaluate the utility of this chemistry and expand the substrate scope,malononitrile and phenylacetonitrile were taken the place of acetonitrile respectively(Fig.4).However,dicyanomethylated quinoline-2,4-dione was not detected.Interestingly,cyanation instead of cyanomethylation occurred while using phenylacetonitrile as the solvent under standard conditions.Phenylacetonitrile has proven to be cyanide anion precursor in the presence of Cu(II)or Pd(II)catalyst[32-33].Based on the previous reports,it was proposed that the cyanation process might proceed via a cyanide radical possibly formed by the oxidation of cyanide anion.So the reaction conditions was optimized to promote the cyanation process.It was found that the conversion could be greatly accelerated by Pd(OAc)2and Cu(OAc)2while using 1.5 equiv.of phenylacetonitrile as the cyanide source and DMF as the solvent.

Fig.4 Radical cyanoalkylation of o-cyanoarylacrylamide with malononitrile and phenylacetonitrile图4邻氰基芳基丙烯酰胺与丙二腈和苯乙腈的自由基氰烷基化
2-(1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydroquinolin-3-yl)acetonitrile(2r):91%yield.1H NMR(600 MHz,Chloroform-d)δ8.02(dd,J=7.8,1.7 Hz,1H),7.69(ddd,J=8.4,7.3,1.7 Hz,1H),7.25-7.19(m,2H),3.51(s,3H),3.12-3.02(m,2H),1.54(s,3H).13C NMR(150 MHz,Chloroform-d)δ193.88,170.69,142.48,136.46,128.24,123.48,119.20,117.02,114.86,55.32,29.96,25.87,21.53.HRMS(ESI):m/z[M+H]+calcd.for C13H13N2O2:229.09770,found 229.09724.
2.4 Mechanism study
To gain insights into the reactionmechanism,control experiments were performed.The presence of TEMPO and BHT hampered the reaction to a great extent,suggesting that a radical intermediate was involved in the catalytic cycle of the reaction.The KIE result of the experiment between CH3CN and CD3CN(kH/kD=6.4)indicated that the sp3C―H bond cleavage of acetonitrile was the ratedetermining step.
Based on our current observations and previous literature reports[16,29],the reaction mechanism was studied preliminarily.Initially,cumyloxyl radical was generated via a copper-assisted homolysis of DCP.Thereafter,the selective hydrogen abstraction from acetonitrile by the cumyloxyl radical generated the corresponding cyanomethyl radical.Subsequently,the addition reaction of the cyanomethyl radical to the activated C=====C bond of arylacrylamide 1 afforded the alkyl radical intermediate A,followed by an intramolecular radical cyclization onto the nitrile group to form the imine radical intermediate B,which finally underwent Habstraction and hydrolysis to form the desired quinoline-2,4-dione 2.

Fig.5 Mechanism study for cyanomethylation of o-cyanoarylacrylamide图5邻氰基芳基丙烯酰胺氰甲基化的机理研究

Fig.6 Proposed mechanism for cyanomethylation of o-cyanoarylacrylamide图6邻氰基芳基丙烯酰胺氰甲基化的反应机理推测
3 Conclusion
In summary,a practical and efficient Cu-mediated tandem radical addition/cyclization ofo-cyanoarylacrylamide with acetonitrile was described.The methodology demonstrated simple operation,mild condition,wide substrate tolerance,providing a convenient access to incorporating cyanomethyl groups into diverse quinoline-2,4-diones.The detailed mechanism and application of the reaction to more complex targets are under investigation in our laboratory.
