Highly Efficient Photothermocatalytic CO2 Reduction in Ni/Mg-Doped Al2O3 with High Fuel Production Rate, Large Light-to-Fuel Efficiency, and Good Durability
2022-07-04XinTanShaowenWuYuanzhiLiQianZhangQianqianHuJichunWuAnZhangandYongdiZhang
Xin Tan, Shaowen Wu, Yuanzhi Li* , Qian Zhang, Qianqian Hu, Jichun Wu, An Zhang, and Yongdi Zhang
1. Introduction
Energy shortage and global warming caused by the combustion of fossil fuels in massive quantities are two major global issues.Photocatalytic reduction in CO2as greenhouse gas with H2O or organic compounds as hole scavengers to synthesize solar fuels by utilizing inexhaustible solar energy affords an exciting strategy to tackle the two issues at the same time, thus having attracted extensive and persistent interests for decades.[1–24]The grand challenges for the photocatalytic strategy to be addressed are to design novel photocatalysts or search for effective approach to efficiently utilizing visible energy,even infrared energy in solar light, considerably raising light-to-fuel efficiency(η)and fuel production rate(rfuel)to satisfy the requirement for potential practical application.[1–24]
Recently, an effective strategy of photothermocatalytic CO2reduction by H2O,[25–30]H2,[31–37]CH4,[38–47]and so on has been developed. The strategy could efficiently utilize UV, visible, and infrared energy in solar light and well combines the high catalytic efficiency of thermocatalysis with light-to-fuel conversion of photocatalysis. rfuelis significantly raised by the strategy.[25–47]In the strategy, photothermocatalytic CO2reduction by CH4(main component of low cost and abundant natural gas)to synthesize syngas (CRM, CO2+ CH4=2CO + 2H2, ΔH298= 247 kJ mol−1, usually known as dry reforming of methane) is especially promising for CO2utilization and solar energy conversion because high rfueland η could be simultaneously acquired.[41–46]It has been reported that supported VIII metal nanoparticles demonstrate catalytic activity for photothermocatalytic CRM.[25–47]Among the catalysts,Ni is attractive due to its excellent photothermocatalytic activity,[40,42–44]inexpensiveness, and earth abundance. For its potential practical application,the key issues to be tackled are to further substantially raise rfueland η,especially improve catalyst durability because Nibased catalysts are prone to deactivation because of the thermodynamically inevitable side reactions of carbon deposition (complete dissociation of CH4and CO disproportionation) accompanying with CRM.[42,48]In principle,the crucial issue of the prone catalyst deactivation is only tackled via kinetically inhibiting carbon deposition, which has been affirmed to be of grand challenge and extremely difficult.Kinetically,the carbon deposition involves the formation of carbon species, and their subsequent polymerization and growth to nanofibers.Therefore, it is imperative to rationally design nanostructured Ni catalysts for inhibiting the kinetic processes of carbon deposition.

Figure 1. XRD patterns of a) Ni/Al2O3, b) Ni/Mg-Al2O3, c) Mg-Al2O3, and d)Al2O3.A)Enlarged XRD patterns of the samples in the range of B)30–50°and C)55–75°.
Herein,we prepared a novel nanocomposite of Ni nanoparticles supported on Mg-doped Al2O3(Ni/Mg-Al2O3). By photothermocatalytic CRM on Ni/Mg-Al2O3merely using focused illumination, high rH2and rCO(69.71 and 74.57 mmol min−1g−1) and extremely large η(32.9%) are acquired. Ni/Mg-Al2O3shows good photothermocatalytic durability owing to carbon deposition being enormously inhibited in comparison with a reference catalyst of Ni nanoparticles loaded on Al2O3(Ni/Al2O3). Based on a number of experimental and theoretic evidences, we reveal that the enormous carbon deposition inhibition is ascribed to the presence of a fence of CO2molecules strongly(strongly adsorbed on Mg-doped Al2O3)around Ni nanoparticles,which accelerates the oxidation of carbon species formed by CH4dissociation, thus blocking the polymerization and growth of carbon species to nanofibers.The high photothermocatalytic activity of Ni/Mg-Al2O3arises from efficient light-driven thermocatalytic CRM which is considerably improved by a novel photoactivation.We put insight into the photoactivation.
2. Results and Discussion
2.1. Characterization
The nanocomposite of Ni nanoparticles loaded on Mg-doped Al2O3(denoted as Ni/Mg-Al2O3) was synthesized by mixing a solution of Mg(NO3)2and Ni(NO3)2with the Al2O3sample prepared by calcination of Al(OH)3, followed by drying at 190 °C and calcining at 500 °C,subsequently by reducing the resultant sample at 700 °C with 5 vol% H2/Ar (detailed in Section 4). For comparison, the reference samples of Ni nanoparticles loaded on Al2O3nanoparticles(denoted as Ni/Al2O3) and Mg-doped Al2O3nanoparticles (denoted as Mg-Al2O3)were prepared (Appendix S1: Experimental). ICP analysis(Appendix S1) demonstrates that the Ni/Al molar ratios in Ni/Mg-Al2O3and Ni/Al2O3are 0.0799 and 0.0623, respectively. The Mg/Al molar ratios in Ni/Mg-Al2O3and Mg-Al2O3are 0.111 and 0.0974,respectively.Figure 1 shows the XRD patterns of the samples.The samples of Ni/Mg-Al2O3, Ni/Al2O3, and Mg-Al2O3have the same XRD patterns as pure Al2O3(Figure 1A), which are assigned to those of cubic crystalline γ-Al2O3(PDF 04-0880). No XRD peaks of crystalline MgO are observed for Ni/Mg-Al2O3and Mg-Al2O3. As can be seen from the enlarged XRD patterns of the samples in the range of 30–50°(Figure 1B) and 55–75° (Figure 1C), the main peaks of Al2O3in Ni/Mg-Al2O3and Mg-Al2O3shift to lower angles.Based on the 2θ data of the peaks,the lattice spacing(d)of Al2O3in the samples was calculated according to the formula (λ = 2dsinθ). As shown in Table S1, the lattice spacings of the corresponding facets of Al2O3in Mg-Al2O3and Ni/Mg-Al2O3have slight expansions as compared to the corresponding those of pure Al2O3. This clearly indicates that Al2O3in Ni/Mg-Al2O3and Mg-Al2O3is doped by Mg2+ion. The lattice expansion of Al2O3induced by Mg doping is attributed to the size of Mg2+ion (0.65 ˚A)being larger than that of Al3+ion (0.5 ˚A). For Ni/Mg-Al2O3and Ni/Al2O3, metallic Ni (PDF 04-0850) is observed (Figure 1A). The average Ni crystal sizes for Ni/Mg-Al2O3and Ni/Al2O3, estimated according to the Scherrer formula at the peak assigned to {200} facet of metallic Ni, are 7.6 and 17.1 nm, respectively. This result shows that Mg-doped Al2O3as support is favorable to the decrease in Ni nanoparticle size as compared to pure Al2O3,of which the mechanism is to be investigated in the future work.
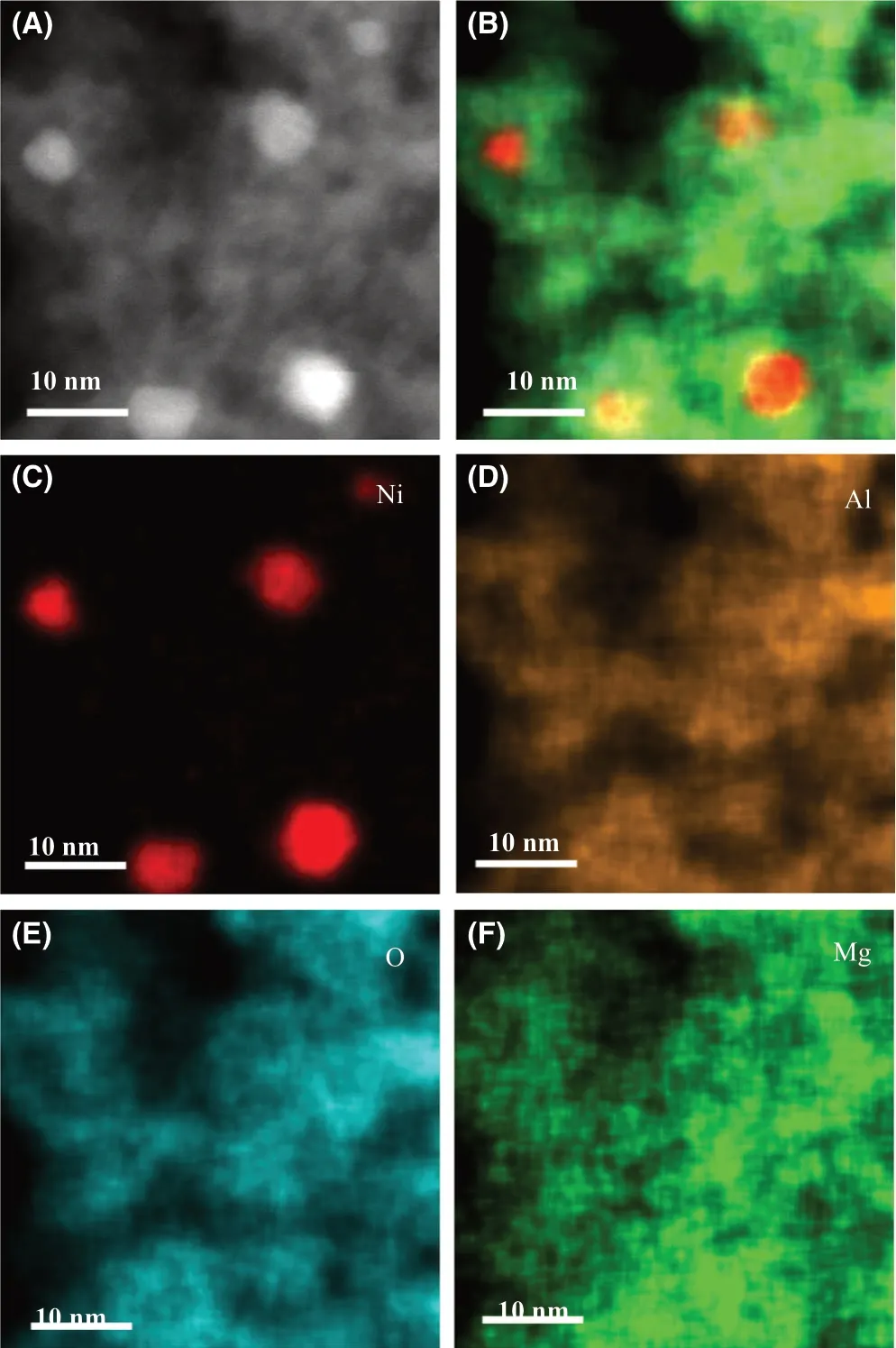
Figure 2. A) HAADF image and the corresponding element mappings of B)all element, C) Ni, D) Al, E) O, and F) Mg of Ni/Mg-Al2O3.
Ni/Mg-Al2O3was characterized by HAADF-STEM and element mappings.As shown in Figure 2A,C,segregated Ni nanoparticles with sizes of 4.6–7.7 nm are observed. The element mappings of Al and O demonstrate that they are uniformly distributed (Figure 2D,E). Mg is uniformly distributed in Al2O3nanoparticles (Figure 2A–D). This observation is consistent with the result by XRD as the Mg doping in Al2O3causes Mg uniform distribution. TEM image of Ni/Mg-Al2O3shows that Ni nanoparticles (black dots) are distributed on Al2O3nanoparticles (Figure S1A). Its HRTEM image demonstrates that Ni nanoparticle with lattice spacing of {111} facet (0.203 nm) is closely contacted to γ-Al2O3nanoparticle with lattice spacing of {321} facet(0.211 nm)(Figure S1B).The results by HAADF-STEM,element mappings, TEM, and HRTEM demonstrate that Ni/Al2O3has morphology of Ni nanoparticles with size of 4.9–17.5 nm supported on Al2O3(Figures S2 and S3).
The samples of Ni/Mg-Al2O3, Ni/Al2O3, Mg-Al2O3, and Al2O3were characterized by XPS (Figure S4). In all the samples, Al and O exist in Al3+and O2−, respectively.[49]For Ni/Mg-Al2O3and Mg-Al2O3, Mg exists in Mg2+. For Ni/Mg-Al2O3and Ni/Al2O3, metallic Ni with binding energy of 852.8 eV[49]is observed, which is consistent with the result of XRD and HRTEM. In addition, Ni2+with binding energy of 855.5 eV[49]is observed. This is ascribed to the surface oxidation of metallic Ni because of the samples of Ni/Mg-Al2O3and Ni/Al2O3being exposed to air.
The Ni/Mg-Al2O3and Ni/Al2O3samples were characterized with nitrogen adsorption (Figures S5 and S6). The BET surface area of Ni/Mg-Al2O3and Ni/Al2O3is 117.1 and 142.0 m2g−1. Their DFT dominant pore sizes are 5.6 and 5.4 nm (Figures S5B and S6B). Their DFT adsorption pore volumes are 0.25 and 0.24 cm3g−1,respectively.
2.2. Photothermocatalytic Activity
Photothermocatalytic CRM on Ni/Mg-Al2O3under focused illumination from a 500 W Xe lamp was evaluated on a home-made reactor with a quartz window.[41,42]No additional heater was used. A feed stream of CH4/CO2/Ar (29.15/28.96/41.89 vol%) continuously flowed at 90.36 mL min−1into the reactor which contained 0.0130 g Ni/Mg-Al2O3. Conspicuously, under focused UV-vis-IR illumination,Ni/Mg-Al2O3shows extremely large photothermocatalytic activity(Figure 3). High reaction rates of CO2(rCO2) and CH4(rCH4) (39.84 and 34.93 mmol min−1g−1) and extremely large production rates of CO (rCO) and H2(rH2) (74.57 and 69.71 mmol min−1g−1) are acquired.The rH2/rCOmolar ratio(0.93)lower than the stoichiometric ratio (1:1) of CRM is attributed to reverse water–gas shift(H2+ CO2= H2O + CO)as a side reaction.[42–45]
CRM is an intense endothermic reaction. Its ΔH298(247 kJ mol−1)approximates that of photocatalytic water splitting(286 kJ mol−1) that is extensively utilized for solar-to-fuel conversion.[50–54]Large rH2and rCO(Figure 3A,B) mean that high efficient light-to-fuel conversion is acquired by photothermocatalytic CRM on Ni/Mg-Al2O3merely using focused UV-vis-IR illumination. Therefore, light-to-fuel efficiency (η)is calculated according to the equation.[41,42,55]

2.3. Photothermocatalytic Durability

Figure 3. A) Reaction rates, B) production rates, and C) light-to-fuel efficiency of Ni/Mg-Al2O3 for photothermocatalytic CRM under focused illumination.
Catalytic durability is of the utmost significance for practical application.The grand challenge for photothermocatalytic CRM is to design a catalyst with good durability because the carbon deposition side reactions are thermodynamically unavoidable.[42,48]The carbon deposition causes not only swift catalyst deactivation,but also blockage in the reactor. Therefore, long-term photothermocatalytic CRM on Ni/Mg-Al2O3was conducted under focused UV-vis-IR illumination. Ni/Mg-Al2O3demonstrates good photothermocatalytic durability (Figure 4A). It is merely during the initial 13 h,the photothermocatalytic activity of Ni/Mg-Al2O3experiences a slight reduction. Within the subsequent 67 h,its photothermocatalytic activity basically keeps unaltered. For comparison,we conducted long-term photothermocatalytic CRM on Ni/Al2O3under the same condition as Ni/Mg-Al2O3. Only after 10-h reaction,the photothermocatalytic activity of Ni/Al2O3considerably decreases(Figure 4A).The quick deactivation of Ni/Al2O3is not due to its larger size of Ni nanoparticles because the catalyst of Ni nanoparticles supported on Al2O3nanosheets with the average Ni crystal size (7.0 nm)similar to that of Ni/Mg-Al2O3(7.6 nm)was reported to quickly deactivate for photothermocatalytic CRM.56This demonstrates that loading Ni nanoparticles on Mg-doped Al2O3considerably promotes the durability as compared to Ni/Al2O3.
To reveal why Ni/Mg-Al2O3has much superior photothermocatalytic durability to Ni/Al2O3, the used samples of Ni/Mg-Al2O3and Ni/Al2O3after the photothermocatalytic durability tests were characterized by TG-MS. The used Ni/Mg-Al2O3sample after reacted for 80 h only demonstrates a weight loss as low as 15.56%because of deposited carbon being combusted (Figure 4B). The carbon deposition rate (rC)of Ni/Mg-Al2O3during the photothermocatalytic durability test was calculated according to the weight loss. Ni/Mg-Al2O3has a low rC(2.3 × 10−3gch−1g−1catalyst). In striking contrast, the used Ni/Al2O3sample after only reacted for 10 h has a high weight loss of 52.75%(Figure S7). Its rCis as high as 0.11 gch−1g−1catalyst(Figure 4C), 48 times larger than that of Ni/Mg-Al2O3. This demonstrates that loading Ni nanoparticles on Mg-doped Al2O3tremendously inhibits carbon deposition,thus improving durability.

Figure 4. A) Time course of reaction and production rates for photothermocatalytic CRM on a) Ni/Mg-Al2O3 and b) Ni/Al2O3 under focused UV-Vis-IR illumination. B) TG-MS profiles of the used Ni/Mg-Al2O3 sample after reacted for 80 h. C) Carbon deposition rates of Ni/Mg-Al2O3 and Ni/Al2O3 during the long-term photothermocatalytic tests.
Why does the carbon deposition not result in obvious deactivation of Ni/Mg-Al2O3like Ni/Al2O3? To address the issue, the used samples of Ni/Mg-Al2O3and Ni/Al2O3after the photothermocatalytic durability tests were further characterized by TEM. TEM images show that carbon nanofibers are observed for both Ni/Mg-Al2O3(Figure S8) and Ni/Al2O3(Figure S9), which is consistent with the results by TG-DSC (Figure 4B and Figure S7B). Interestingly, HRTEM image shows that for the used Ni/Mg-Al2O3sample, Ni nanocrystal still demonstrates clear lattice spacing of {111} facet such as fresh Ni/Mg-Al2O3(Figure S8B,C). This shows that the catalytically active sites on Ni nanocrystal surface are not covered by the deposited carbon species. This accounts for the good photothermocatalytic durability of Ni/Mg-Al2O3(Figure 4A). In striking contrast, Ni nanocrystal for the used Ni/Al2O3sample is covered by thick layers of graphite carbon ({111} facet, 0.335 nm, PDF#75-2078) (Figure S9B,C). The lattice spacing of Ni nanocrystal becomes blurred owing to the coverage of carbon species on Ni nanocrystal surface (Figure S9B). The deposited carbon blocks the access of reactants to the catalytically active sites on Ni nanocrystal surface, thus causing the deactivation of Ni/Al2O3(Figure 4A).

Figure 5. The CO2-TPO-C profiles of Ni/Mg-Al2O3 and Ni/Al2O3 in the dark: A) CO2 consumption, B) CO and C) H2.
2.4. Origin of Carbon Deposition Inhibition

Figure 6. CO2 adsorption isotherms of A) Ni/Mg-Al2O3 and B) Ni/Al2O3 at 0 °C. C) TPD-CO2 profiles of the samples in the dark. Note: In Figure 6C,Al2O3 has CO2 desorption profile the almost same as that of Ni/Al2O3,while Mg-Al2O3 has CO2 desorption profile the almost same as that of Ni/Mg-Al2O3. The results demonstrate that CO2 molecules are mainly adsorbed on Al2O3 or Mg-Al2O3 rather than Ni nanoparticles.
The reported theoretic studies (DFT) reveal that CRM on metallic Ni involves the elementary steps of step-by-step dissociations of CH4to CHx* and H*, CO2dissociation to O* and CO*, and CHx* oxidation by O*.[42,44,57]Among the steps, CH* dissociation to C* and the oxidation of C*and CH*by O*are the rate-determining steps because of their high activation energy.[42,44,57]The steps of C*and CH*oxidation not only decide the reaction rate,but also affect the carbon deposition rate, thus affecting the catalytic durability. To put insight in the origin why loading Ni nanoparticles on Mg-doped Al2O3(Ni/Mg-Al2O3) enormously reduces the carbon deposition rate in comparison with Ni/Al2O3, we performed CO2temperature-programmed oxidation of carbon species (CO2-TPO-C) on Ni/Mg-Al2O3and Ni/Al2O3produced by pre-dissociation of CH4(Appendix S1: Experimental).As the rate-determining step of CH* by O* to CHO* is followed by the step of CHO* dissociation to CO* and H*,[42,44,57]both the produced CO and H2during CO2-TPO-C were monitored and quantitatively analyzed by MS (Appendix S1: Experimental). For Ni/Al2O3, an intense CO2consumption peak is shown around 691 °C(Figure 5A), accompanying with a strong CO peak (Figure 5B) and weak H2peak (Figure 5C). The formation of CO is due to CHx*species (x = 0–3) (produced by the pre-dissociation of CH4) being oxidized by CO2. The formation of H2is due to the subsequent dissociation of the oxidation intermediates of CHx* species (x = 1–3).The specific amount of produced CO and H2is 117.2 and 0.78 mmol g−1catalyst, respectively. This demonstrates that a considerable amount of carbon species are deposited on Ni/Al2O3during the pre-dissociation of CH4, which is consistent with the result by TG-MS (Figure 4C and Figure S7B). The much higher specific amount of CO than H2indicates that the produced CO during CO2-TPO-C mainly arises from C* oxidation. In striking contrast, for Ni/Mg-Al2O3, there is weak CO2consumption peak around 605 °C(Figure 5A), accompanying with a weak CO peak (Figure 5B) and H2peak (Figure 5C). The specific amount of produced CO and H2is 11.6 and 7.94 × 10−2mmol g−1catalyst, respectively. This demonstrates that only a very small amount of carbon species is deposited on Ni/Mg-Al2O3during the pre-dissociation of CH4,which is consistent with the result by TG-MS (Figure 4B,C). The much higher specific amount of CO than H2indicates that the produced CO during CO2-TPO-C for Ni/Mg-Al2O3also mainly arises from C* oxidation. It is worth mentioning that the peak temperature of carbon species being oxidized by CO2for Ni/Mg-Al2O3significantly shifts from 691 °C (Ni/Al2O3) to 605 °C. This demonstrates that compared to Ni/Al2O3, loading Ni nanoparticles on Mg-doped Al2O3considerably accelerates the oxidation of carbon species, thus enormously reducing carbon deposition rate (Figure 4C).
The acceleration of carbon species oxidation is attributed to more CO2molecules being strongly adsorbed on Mg-doped Al2O3as compared to Al2O3. This is verified by CO2adsorption and temperatureprogrammed desorption of CO2(TPD-CO2) pre-adsorbed on Ni/Mg-Al2O3and Ni/Al2O3(Appendix S1:Experimental).Figure 6A,B shows CO2adsorption isotherms of the Ni/Mg-Al2O3and Ni/Al2O3samples at 0 °C (ice water), respectively. Based on the adsorption data in Figure 6A,B,CO2monolayer saturation adsorption capacities(Q)were calculated by BET method through plotting P/P0versus 1/[Q(P0/P −1)].Good linear relationship between P/P0and 1/[Q(P0/P −1)] is observed (Figure S10). Q of Ni/Mg-Al2O3is 63.3 cm3g−1(STP),which is much higher than that of Ni/Al2O3(32.1 cm3g−1(STP)).The result indicates that the Mg2+doping significantly increases the CO2adsorption capacity of Al2O3.
TPD-CO2shows that Ni/Al2O3has a strong peak of CO2desorption around 85 °C (Figure 6C), which is attributed to CO2molecules being adsorbed on the basic sites of alumina.[58]CO2desorption extends up to 270 °C,indicating the presence of different types of basic sites on Al2O3that strongly adsorb CO2. Compared to Ni/Al2O3, the CO2desorption peak for Ni/Mg-Al2O3around 85 °C is considerably intensified (Figure 6C). Moreover,CO2desorption extends up to higher temperature (380 °C). The result indicates that the Mg2+doping significantly increases the CO2adsorption capacity and strength of Al2O3.
Based on the results of Characterization (Section 2.1), CO2-TPOC, CO2adsorption, and TPD-CO2, a mechanism of carbon inhibition for CRM on Ni/Mg-Al2O3as compared to Ni/Al2O3is proposed (Scheme 1). For Ni/Al2O3, CH4molecules dissociate to hydrogen and carbon species, and CO2molecules dissociate to CO and oxygen on Ni nanoparticles. Carbon species are oxidized by oxygen to CO (Scheme 1A). On the other hand, carbon species migrate, polymerize, and finally grow to nanofibers(Scheme 1A). The side reaction leads to severe carbon deposition (Figures S7 and S9), thus causing the deactivation of Ni/Al2O3. For Ni/Mg-Al2O3, CO2molecules strongly adsorb on Mg-doped Al2O3(Figure 5C), forming a CO2molecule fence around Ni nanoparticle (Scheme 1B). Carbon species migrate to the interface between Ni nanoparticle and Mg-doped Al2O3,where they are oxidized to CO by the strongly adsorbed CO2molecules (Scheme 1B). This blocks the polymerization and growth of carbon species to nanofibers, thus enormously reducing carbon deposition and increasing catalytic durability(Figure 4).
To further corroborate the mechanism, we calculated the activation energy of the migration of C* and CH* (the carbon species associated with the rate-determining steps of CRM[42,44,57])on metallic Ni.A slab of Ni36with cubic structure (PDF 04-0850) and 3 × 3 {111} surface was constructed.The{111}surface was chosen because Ni nanoparticle has lattice spacing of{111}facet(0.203 nm)(Figure S1B).The configurations of C*and CH*adsorbed on different sites are optimized(Figure S11).The transition state of C* or CH* being migrated from one site to another site was determined by using Dimer method.[42,44,57]The activation energies (Ea) of C* and CH* migration are 0.32 and 0.34 eV,respectively. The very low Eavalues suggest that carbon species can easily migrate from Ni nanoparticles to the Ni/Mg-Al2O3interface,where they are oxidized by CO2strongly adsorbed on Mg-doped Al2O3(Figure 5,Scheme 1B).
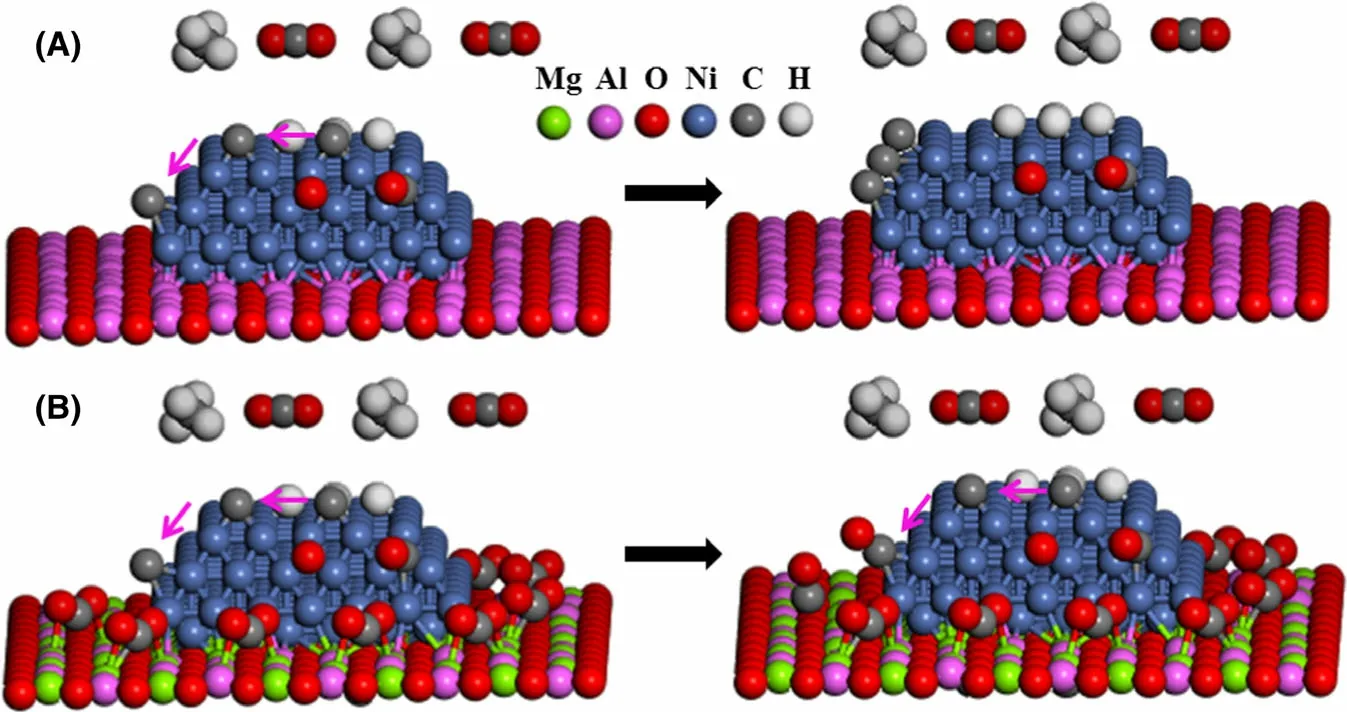
Scheme 1. Schematic illustration of the mechanism of A) carbon deposition for Ni/Al2O3 and B)carbon deposition inhibition induced by a fence of CO2 molecules (strongly adsorbed on Mg-doped Al2O3) around Ni nanoparticle for Ni/Mg-Al2O3.
2.5. Roles of Light
2.5.1. Heating: Light-Driven Thermocatalysis
To disclose why CRM efficiently proceeds on Ni/Mg-Al2O3upon the focused illumination,we recorded the optical absorption spectra of the samples. Ni/Mg-Al2O3has a strong absorption across whole solar spectra (Figure 7A). This is attributed to the strong surface plasmonic absorption of Ni nanoparticles,[42,44]the optical absorption of Ni nanoparticles induced by near-field dielectric scattering of support (e.g., Mg-Al2O3in our case),[59]and the absorption of Mg-Al2O3(Figure 7A). Ni/Al2O3has absorption spectra similar to Ni/Mg-Al2O3, indicating that the Mg doping does not obviously alter the optical absorption property.However, the photocatalytic test at near room temperature(Appendix S1: Experimental) demonstrates that under focused UVvis-IR illumination, Ni/Mg-Al2O3has no photocatalytic activity for CRM (Figure 7B). This shows that the highly efficient photothermocatalytic CRM on Ni/Mg-Al2O3(Figure 3) originates from lightdriven thermocatalysis.
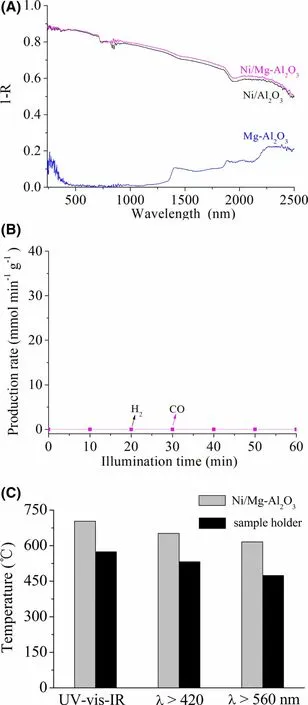
Figure 7. A) Absorption spectra of Ni/Mg-Al2O3, Ni/Al2O3, and Mg-Al2O3(R is the reflectance). B) Time course of rH2 and rCO for photocatalytic CRM on Ni/Mg-Al2O3 at near ambient temperature. C) The Teq values of Ni/Mg-Al2O3 and the sample holder under photothermocatalytic reaction conditions with focused illumination (Figure 3). The sample holder has high Teq values because of the IR heating effect of focused illumination.
The focused illumination causes a quick elevation in the surface temperature of Ni/Mg-Al2O3to an equilibrium temperature (Teq),which is ascribed to the IR heating effect of the focused illumination (Figure 7C) and photothermal conversion because of its strong optical absorption (Figure 7A). Once the Teqvalue surpasses the light-off temperature (Tlight-off) at which thermocatalytic CRM on Ni/Mg-Al2O3is triggered, light-driven thermocatalytic CRM takes place.
To corroborate the mechanism, Teqand Tlight-offof Ni/Mg-Al2O3were measured under the photothermocatalytic conditions (Figure 3).Under focused illumination of UV-vis-IR,λ > 420 and 560 nm vis-IR,the Teqvalues of Ni/Mg-Al2O3are 703 °C, 652 °C, and 616 °C,respectively (Figure 7C). The thermocatalytic test in the dark(Appendix S1: Experimental) demonstrates that Tlight-offfor thermocatalytic CRM on Ni/Mg-Al2O3is about 450 °C (Figure 8A). All the Teqvalues of Ni/Mg-Al2O3surpass its Tlight-off.Therefore,light-driven thermocatalytic CRM on Ni/Mg-Al2O3takes place.

Figure 8. The values of A) rCH4 and B) rCO2 versus T, and C) Ln (rCH4)versus 1/T for CRM on Ni/Mg-Al2O3 under focused illumination and in the dark.
2.5.2. Photoactivation
To address the issue if light merely acts as a heating role for photothermocatalytic CRM on Ni/Mg-Al2O3, catalytic CRM on Ni/Mg-Al2O3under focused illumination and in the dark at different temperatures (Appendix S1: Experimental) was conducted. Compared to those in the dark,the rCO2and rCH4are considerably raised upon focused UV-vis-IR illumination at the same temperature above 425 °C (Figure 8A,B). Upon λ > 420 and 560 nm focused vis-IR illumination, rCH4and rCO2are considerably enhanced (Figure 8A,B). As Ni/Mg-Al2O3does not demonstrate photocatalytic activity (Figure 7B), the catalytic enhancement upon the illumination shows the presence of a photoactivation for photothermocatalytic CRM on Ni/Mg-Al2O3.

Figure 9. A) TPD-CH4 and B) CO2-TPO-C profiles of Ni/Mg-Al2O3 under focused illumination and in the dark.
To further disclose the photoactivation,we plotted Figures of Ln(rCH4) versus 1/T according to the data of Ni/Mg-Al2O3in Figure 8A.Ln(rCH4)versus 1/T demonstrates a well linear relationship(Figure 8C). The apparent activation energy (Ea,ap) was calculated in accordance with Arrhenius equation (k=Ae-Ea/RT). In the dark,Ni/Mg-Al2O3has an Ea,apvalue of 80.3 kJ mol−1. Focused illumination of UV-vis-IR, λ > 420, and 560 nm vis-IR leads to a considerable reduction in the Ea,apvalues of Ni/Mg-Al2O3to 45.7,48.5, and 52.5 kJ mol−1, respectively. This demonstrates that the illumination considerably reduces the Ea,apvalues, thus raising photothermocatalytic activity.
As discussed on Section 2.4, CH* dissociation to C* is one of the rate-determining steps for CRM on metallic Ni.[42,44,57]It means that the dissociations of inert CH4molecules play a decisive role for CRM on Ni/Mg-Al2O3.To verify whether CH4dissociation is affected by the photoactivation, temperature-programmed dissociation of CH4(TPD-CH4)on Ni/Mg-Al2O3under focused UV-vis-IR illumination and in the dark was conducted (Appendix S1:Experimental).In the dark,CH4dissociation occurs around 566 °C(Figure 9A). The peak temperature of CH4dissociation substantially shifts to 523 °C under focused UV-vis-IR illumination. This indicates that the photoactivation promotes CH4dissociation, thus accelerating photothermocatalytic activity of Ni/Mg-Al2O3. DFT calculations show that for CRM on a slab of Ni36with {111} surface,the activation energy of CH* dissociation to C* under excited state (1.26 eV, under irradiation) is obviously lower than that in the ground state(1.37,in the dark).[60]Recently,Zhou et al reveal by using DFT calculations that the activation energy of CH* dissociation to C* under excited states is obviously lower than that in the ground state for CRM on a slab of RuCu6.[46]Our result provides the experimental evidence to the theoretic results by DFT calculations.
As discussed above,the oxidations of CH*and C*species are also the rate-determining steps for CRM on metallic Ni.[42,44,57]To verify whether the oxidation steps are affected by the photoactivation,CO2temperature-programmed oxidation of carbon species(CO2-TPO-C)on Ni/Mg-Al2O3(produced by pre-dissociation of CH4)under focused UV-vis-IR illumination and in the dark was conducted(Appendix S1:Experimental).In the dark,the oxidation of CHx(x = 0–3)species occurs around 609 °C,accompanying with a strong peak of CO production(Figure 9B)and a weak peak of H2production due to the subsequent dissociation of the oxidation intermediates of CHx(x = 1–3) as discussed above(Figure 9C). The CO and H2peaks obviously shift to lower temperature(591 °C)under focused UV-vis-IR illumination.This indicates that the photoactivation promotes the oxidation of carbon species produced by CH4dissociation.The promotion of carbon species oxidation is favorable not only for accelerating photothermocatalytic activity of Ni/Mg-Al2O,but also for decreasing carbon deposition rate, thus promoting photothermocatalytic durability.
3. Conclusions
In summary, Ni/Mg-Al2O3demonstrates high production rates of H2and CO for photothermocatalytic CRM merely using focused illumination. An extremely large light-to-fuel efficiency of 32.9% is acquired.Ni/Mg-Al2O3shows excellent photothermocatalytic durability owing to carbon deposition being enormously inhibited in comparison with Ni/Al2O3. The enormous carbon deposition inhibition is ascribed to the presence of a fence of CO2molecules (strongly adsorbed on Mgdoped Al2O3) around Ni nanoparticles. The CO2molecule fence reacts with carbon species produced by CH4dissociation on Ni nanoparticles,consequently blocks the polymerization and growth of carbon species to nanofibers, thus significantly inhibiting carbon deposition. The highly efficient photothermocatalytic CRM on Ni/Mg-Al2O3is attributed to efficient light-driven thermocatalytic CRM. A photoactivation is found to accelerate the rate-determining steps of CRM (e.g., methane dissociation and the oxidation of carbon species), thus considerably enhancing photothermocatalytic activity of Ni/Mg-Al2O3. The present work provides a promising strategy of designing a catalyst with high photothermocatalytic activity and excellent durability for highly efficient CO2reduction and solar-to-fuel conversion.
4. Experimental Section
Preparation: 6.12 g Al(OH)3powder in a crucible was heated at a rate of 5 °C min−1to 500 °C and maintained at 500 °C for 4 h in a muffle furnace.The resultant Al2O3sample was denoted as Al2O3. 0.2852 g Ni(NO3)2∙6H2O and 0.5030 g Mg(NO3)2∙6H2O were dissolved in 5 mL deionized water in a beaker.After magnetic stirring for 10 min, the solution was mixed with 1.0 g Al2O3in a ceramic crucible. The crucible was placed in an electric heating jacket, heated to 190 °C.The mixture was ground with an agate pestle until water was completely evaporated.The obtained powder was heated at 5 °C min−1to 500 °C,and maintained at 500 °C for 8 h in a muffle furnace.The resultant sample was denoted as NiO/Mg-Al2O3. 0.10 g of NiO/Mg-Al2O3, put in a quartz tubular reactor, was heated at 10 °C min−1to 700 °C and maintained at 700°C for 1 h in a flow 5 vol%H2/Ar(30 mL min−1).The obtained sample was labeled as Ni/Mg-Al2O3.
The Mg-doped Al2O3sample (denoted as Mg-Al2O3) was prepared by the same procedure as that of NiO/Mg-Al2O3except for no addition of Ni(NO3)2∙6H2O.
Acknowledgements
This work was supported by National Natural Science Foundation of China(21972109, 21673168). DFT calculations were conducted at the Shanghai Supercomputer Center(SSC),China.
Conflict of Interest
The authors declare no conflict of interest.
Supporting Information
Supporting Informationis available from the Wiley Online Library or from the author.
Keywords
CO2reduction, light-to-fuel efficiency, Ni/Mg-Al2O3nanocomposite,photocatalytic, photothermocatalytic
Received: January 26, 2021
Revised: March 22, 2021
Published online: March 24, 2021
[1] X. Li, J. G. Yu, M. Jaroniec, X. B. Chen, Chem. Rev. 2019, 119, 3962.
[2] Z. Jiang, X. H. Xu, Y. H. Ma, H. S. Cho, D. Ding, C. Wang, J. Wu, P. Oleynikov, M. Jia, J. Cheng, Y. Zhou, O. Terasaki, T. Y. Peng, L. Zan, H. X.Deng, Nature 2020, 586, 549.
[3] K. Q. Lu, Y. H. Li, F. Zhang, M. Y. Qi, X. Chen, Z. R. Tang, Y. M. A.Yamada, M. Anpo, M. Conte, Y. J. Xu, Nat. Commun. 2020, 11, 5181.
[4] B. Han, X. W. Ou, Z. Q. Deng, Y. Song, C. Tian, H. Deng, Y. J. Xu, Z. Lin,Angew. Chem. Int. Ed. 2018, 57, 16811.
[5] Y. G. Wang, T. Li, Y. G. Yao, X. Li, X. Bai, C. H. Yin, N. Williams, S. F.Kang, L. F. Cui, L. B. Hu, Adv. Energy Mater. 2018, 8, 1703136.
[6] F. Raziq, L. Q. Sun, Y. Y. Wang, X. L. Zhang, M. Humayun, S. Ali, L. L.Bai, Y. Qu, H. T. Yu, L. Q. Jing, Adv. Energy Mater. 2018, 8, 1701580.
[7] C. Dong, C. Lian, S. Hu, Z. Deng, J. Gong, M. Li, H. Liu, M. Xing, J.Zhang, Nat. Commun. 2018, 9, 1252.
[8] F. Chen, Z. Ma, L. Ye, T. Ma, T. Zhang, Y. Zhang, H. Huang, Adv. Mater.2020, 32, 1908350.
[9] Z.F.Jiang,H.L.Sun,T.Q.Wang,B.Wang,W.Wei,H.M.Li,S.Q.Yuan,T.C.An,H.J.Zhao,J.G.Yu,P.K.Wong,Energy Environ.Sci.2018,11,2382.
[10] S. Shoji, X. Peng, A. Yamaguchi, R. Watanabe, C. Fukuhara, Y. Cho, T.Yamamoto, S. Matsumura, M.-W. Yu, S. Ishii, T. Fujita, H. Abe, M.Miyauchi, Nat. Catalysis 2020, 3, 148.
[11] C. Bie, B. Zhu, F. Xu, L. Zhang, J. Yu, Adv. Mater. 2019, 31, 1902868.
“打虎”反腐产生了良好的震慑效果,社会清流不断涌现。在中国社会风气日渐好转的时代背景下,党的十九大审时度势,提出由打存量“老虎”为主转向拍增量“苍蝇”为主,打击基层腐败已然成为党和国家新的战略任务,有助于基层和农村形势好转,这也将成为未来中国农业和农村发展的新景。
[12] Y. He, H. Rao, K. Song, J. Li, Y. Yu, Y. Lou, C. Li, Y. Han, Z. Shi, S. Feng,Adv. Funct. Mater. 2019, 29, 1905153.
[13] F. Xu, K. Meng, B. Zhu, H. Liu, J. Xu, J. Yu, Adv. Funct. Mater. 2019, 29,1904256.
[14] L. Zeng, Z. Wang, Y. Wang, J. Wang, Y. Guo, H. Hu, X. He, C. Wang, W.Lin, J. Am. Chem. Soc. 2020, 142, 75.
[15] L. Wang, J. Wan, Y. Zhao, N. Yang, D. Wang, J. Am. Chem. Soc. 2019,141, 2238.
[16] Z. B. Fang, T. T. Liu, J. Liu, S. Jin, X. P. Wu, X. Q. Gong, K. Wang, Q. Yin,T. F. Liu, R. Cao, H. C. Zhou, J. Am. Chem. Soc. 2020, 142, 12515.
[17] X. Ren, M. Gao, Y. Zhang, Z. Zhang, X. Cao, B. Wang, X. Wang, Appl.Catal. B. 2020, 274, 119063.
[18] S. B. Wang, B. Y. Guan, X. W. Lou, Energy. Environ. Sci. 2018, 11, 306.
[19] J. Liang, Y. Chai, L. Li, D. Li, J. Shen, Y. Zhang, X. Wang, Appl. Catal. B.2020, 265, 118551.
[20] H. Yang, D. Yang, X. Wang, Angew. Chem. Int. 2020, 59, 15527.
[21] S. Sorcar, Y. H. Wang, J. Lee, H. Kim, K. M. Grimes, C. A. Grimes, J. W.Jung, C. H. Cho, T. Majima, M. R. Hoffmannd, S. In, Energy Environ. Sci.2019, 12, 2685.
[22] Q. G. Zhai, S. J. Xie, W. Q. Fan, Q. H. Zhang, Y. Wang, W. P. Deng, Y.Wang, Angew. Chem. 2013, 125, 5888.
[23] L. Lu, B. Wang, S. M. Wang, Z. Shi, S. C. Yan, Z. G. Zou, Adv. Funct.Mater. 2017, 27, 1702447.
[24] W.N. Wang, W. J. An,B. Ramalingam,S. Mukherjee,D. M. Niedzwiedzki,S.Gangopadhyay,P.Biswas,J.Am.Chem.Soc.2012,134,11276.
[25] Y. Y. Li, C. H. Wang, M. Song, D. S. Li, X. T. Zhang, Y. C. Liu, Appl.Catal. B. 2019, 243, 760.
[26] J. Yan, C. Wang, H. Ma, Y. Li, Y. Liu, N. Suzuki, C. Terashima, A.Fujishima, X. T. Zhang, Appl. Catal. B. 2020, 268, 118401.
[27] X. Chen, Q. Li, J. Li, J. Chen, H. Jia, Appl. Catal. B. 2020, 270, 118915.
[28] P. Li, L. Liu, W. An, H. Wang, H. Guo, Y. Liang, W. Cui, Appl. Catal. B.2020, 266, 118618.
[29] L. Zhang, Y. P. Meng, J. S. Tian, L. J. Zhang, S. L. Wan, J. D. Lin, Y. Wang,Chemsuschem 2017, 10, 4709.
[30] M. N. Ha, G. Z. Lu, Z. F. Liu, L. C. Wang, Z. Zhao, J. Mater. Chem. A 2016, 4, 13155.
[31] L. B. Hoch, P. G. O’Brien, A. Jelle, A. Sandhel, D. D. Perovic, C. A. Mims,G. A. Ozin, ACS Nano 2016, 10, 9017.
[32] J. Ren, S. X. Ouyang, H. Xu, X. G. Meng, T. Wang, D. F. Wang, J. H. Ye,Adv. Energy Mater. 2017, 7, 1601657.
[33] L. Wang, M. Ghoussoub, H. Wang, Y. Shao, W. Sun, A. A. Tountas, T. E.Wood, H. Li, J. Y. Y. Loh, Y. Dong, M. Xia, Y. Li, S. Wang, J. Jia, C. Qiu,C. Qian, N. P. Kherani, L. He, X. Zhang, G. A. Ozin, Joule 2018, 2, 1369.
[34] W. B. Zhang, L. B. Wang, K. W. Wang, M. U. Khan, M. L. Wang, H. L. Li,J. Zeng, Small 2017, 13, 1602583.
[35] K. Feng, S. Wang, D. Zhang, L. Wang, Y. Yu, K. Feng, Z. Li, Z. Zhu, C. Li,M. Cai, Z. Wu, N. Kong, B. Yan, J. Zhong, X. Zhang, G. A. Ozin, L. He,Adv. Mater. 2020, 32, 2000014.
[36] Y. Fang, K. Lv, Z. Li, N. Kong, S. Wang, A. B. Xu, Z. Wu, F. Jiang, C. Li,G. A. Ozin, L. He, Adv. Sci. 2020, 7, 2000310.
[37] G. Chen, R. Gao, Y. Zhao, Z. Li, G. I. N. Waterhouse, R. Shi, J. Zhao, M.Zhang, L. Shang, G. Sheng, X. Zhang, X. Wen, L. Z. Wu, C. H. Tung, T.R. Zhang, Adv. Mater. 2018, 30, 1704663.
[38] B. Han, W. Wei, L. Chang, P. F. Cheng, Y. H. Hu, ACS Catal. 2016, 6, 494.
[39] H. M. Liu, X. G. Meng, T. D. Dao, H. B. Zhang, P. Li, K. Chang, T. Wang,M. Li, T. Nagao, J. H. Ye, Angew. Chem. Int. Ed. 2015, 54, 11545.
[40] H. M. Liu, T. D. Dao, L. Q. Liu, X. G. Meng, T. Nagao, J. H. Ye, Appl.Catal. B. 2017, 209, 183.
[41] M. Y. Mao, Q. Zhang, Y. Yang, Y. Z. Li, H. Huang, Z. K. Jiang, Q. Q. Hu,X. J. Zhao, Green Chem. 2018, 20, 2857.
[42] H. Huang, M. Y. Mao, Q. Zhang, Y. Z. Li, J. L. Bai, Y. Yang, M. Zeng, X.J. Zhao, Adv. Energy Mater. 2018, 8, 1702472.
[43] G. Q. Zhang, S. W. Wu, Y. Z. Li, Q. Zhang, Appl. Catal. B. 2020, 264,118544.
[44] S. W. Wu, Y. Z. Li, Q. Zhang, Q. Q. Hu, J. C. Wu, C. Y. Zhou, X. J. Zhao,Adv. Energy Mater. 2020, 10, 2002602.
[45] S. W. Wu, Y. Z. Li, Q. Zhang, Z. K. Jiang, Y. Yang, J. C. Wu, X. J. Zhao,Energy Environ. Sci. 2019, 12, 2581.
[46] L. Zhou, J. M. P. Martirez, J. Finzel, C. Zhang, D. F. Swearer, S. Tian, H.Robatjazi, M. Lou, L. Dong, L. Henderson, P. Christopher, E. A. Carter, P.Nordlander, N. J. Halas, Nat. Energy 2020, 5, 61.
[47] F. Pan, X. M. Xiang, Z. C. Du, E. Sarnello, T. Li, Y. Li, Appl. Catal. B.2020, 260, 118189.
[48] J. G. Zhang, H. Wang, A. K. Dalai, J. Catal. 2007, 249, 300.
[49] C. D. Wagner, W. M. Riggs, L. E. Davis, Handbook of X-ray Photoelectron Spectroscopy, PerkinElmer, Waltham, MA 1979.
[50] Y. Wang, H. Suzuki, J. Xie, O. Tomita, D. J. Martin, M. Higashi, D. Kong,R. Abe, J. W. Tang, Chem. Rev. 2018, 118, 5201.
[51] J. S. Xu, C. Yang, S. Bi, W. Y. Wang, Y. F. He, D. Q. Wu, Q. F. Liang, X.C. Wang, F. Zhang, Angew. Chem. Intern. Ed. 2020, 59, 23845.
[52] J. R. Ran, J. T. Qu, H. P. Zhang, T. Wen, H. L. Wang, S. M. Chen, L. Song,X. L. Zhang, L. Q. Jing, R. K. Zheng, S. Z. Qiao, Adv. Energy Mater. 2019,9, 1803402.
[53] L. Liao, Q. H. Zhang, Z. H. Su, Z. Z. Zhao, Y. N. Wang, Y. Li, X. X. Lu,D. G. Wei, G. Y. Feng, Q. K. Yu, X. J. Cai, J. M. Zhao, Z. F. Ren, H.Fang, F. Robles-Hernandez, S. Baldelli, J. M. Bao, Nat. Nanotechnol.2014, 9, 69.
[54] J. Liu, Y. Liu, N. Y. Liu, Y. Z. Han, X. Zhang, H. Huang, Y. Lifshitz, S. T.Lee, J. Zhong, Z. H. Kang, Science 2015, 347, 970.
[55] D. Marxer, P. Furler, M. Takacs, A. Steinfeld, Energy Environ. Sci. 2017,10, 1142.
[56] Q. Zhang, Y. Z. Li, S. W. Wu, J. C. Wu, Z. K. Jiang, Y. Yang, L. Ren, X. J.Zhao, J. Mater. Chem. A 2019, 7, 19800.
[57] Y. A. Zhu, D. Chen, X. G. Zhou, W. K. Yuan, Catal. Today 2009, 148, 260.
[58] S. Carre, B. Tapin, N. S. Gnep, R. Revel, P. Magnoux, Appl. Catal. A 2010, 372, 26.
[59] N. Zhang, C. Han, Y. J. Xu, J. J. Foley IV, D. T. Zhang, J. Codrington, S. K.Gray, Y. G. Sun, Nat. Photon. 2016, 10, 473.
[60] Q. Zhang, M. Y. Mao, Y. Z. Li, Y. Yang, H. Huang, Z. K. Jiang, Q. Q. Hu,S. W. Wu, X. J. Zhao, Appl. Catal. B 2018, 239, 555.
猜你喜欢
杂志排行
Energy & Environmental Materials的其它文章
- Progress of Pb-Sn Mixed Perovskites for Photovoltaics:A Review
- Development Strategies in Transition Metal Borides for Electrochemical Water Splitting
- Polymer-/Ceramic-based Dielectric Composites for Energy Storage and Conversion
- Controllable Construction of Bifunctional CoxP@N,P-Doped Carbon Electrocatalysts for Rechargeable Zinc–Air Batteries
- Unveiling the Underlying Mechanism of Transition Metal Atoms Anchored Square Tetracyanoquinodimethane Monolayers as Electrocatalysts for N2 Fixation
- Rational Design of High-Performance Bilayer Solar Evaporator by Using Waste Polyester-Derived Porous Carbon-Coated Wood
