Polysaccharides Based Random and Unidirectional Aerogels for Thermal and Mechanical Stability
2022-05-09CHAUDARYAneebaCHUDHARYTaybaFAROOQAmjadZHANGMeiling张美玲PATOARYMohammedKayesLIULifang刘丽芳
CHAUDARY Aneeba, CHUDHARY Tayba, FAROOQ Amjad, ZHANG Meiling(张美玲), PATOARY Mohammed Kayes, LIU Lifang(刘丽芳)*
1 College of Textiles, Donghua University, Shanghai 201620, China2 Key Laboratory of Textile Science & Technology, Ministry of Education, Donghua University, Shanghai 201620, China3 Department of Chemistry, Zhejiang University, Hangzhou 310027, China
Abstract: Owing to the increasing energy demands and the environmental constraints, the need for bio-based materials has been on the rise due to their variety of favorable properties like biocompatibility, cost-effectiveness, large specific surface area, high porosity, and non-toxicity. Thermal stability and mechanical strength of aerogels are highly dependent on their micro-porous structures. A three-dimensional structure based on cellulose nanofiber/chitosan (CNF/CS) aerogels was built using two different freezing methodologies, namely random freezing, and unidirectional freezing techniques, by changing mold shapes. The unidirectional aerogels ultimately resulted in high-temperature stability and mechanical strength. The results show that the unidirectional CNF/CS(u-CNF/CS) aerogels contain controlled micro porous orientation relative to random-CNF/CS (r-CNF/CS) aerogels with the disordered porous distribution. The high-temperature stability with an increase of glass transition temperature Tg from 275 ℃ (CNF) to 283 ℃ (CNF/CS), the ultra-low thermal conductivity of 0.030 W/(m ·K), and mechanical robustness of u-CNF/CS aerogels make them quite favorable for practical applications.
Key words: aerogel; cellulose nanofiber (CNF); chitosan (CS); thermal stability; mechanical robustness
Introduction
Increasing thermal insulation demand causes huge energy loss in greenhouse gas emissions. To address this thermal insulation need, many porous materials have been introduced because of their lowest thermal insulation behavior. Mineral wood and polymers with their best thermal conductivity about 0.030-0.040 W/(m ·K) have been commercially popular lately[1]. Vacuum insulation panels contain the lowest thermal insulation about 0.007-0.008 W/(m ·K), but they are costly with poor mechanical stability[1]. Aerogels with the best thermal and mechanical performance are well known in superinsulation fields because of their mesoporosity (≥ 90%) and the lowest density (about 0.1 g/cm3).
Polysaccharides, as abundantly available renewable carbohydrate polymers, hold abundant characteristics, such as biodegradability, non-toxicity, environment friendliness, and presence of large functional groups for example carboxylic groups and amino groups which help to form polysaccharides derivatives[2-3]. Cellulose nanofiber (CNF) and chitosan (CS) are natural polysaccharides with abundant presence in plants, fungi, and bacteria-containing amorphous and crystalline domains. Polysaccharides are carbohydrates of polymers in which glycosidic bonds are present[4-6].
Cellulose has gained significant interest in the past years due to holding outstanding properties: renewability, biocompatibility, the lowest density, surface reactivity[7], good thermal properties[8-11], and mechanical characteristics[12]. CNF suspension holds gel-like properties at low concentrations[13]. However, the rheological properties of CNF depend on CNF concentration and pH[14-15]. CS adds strength to the structure when mixed in an aqueous form with another polysaccharide, and gives good structure stability with minimizing structural defects within the geometry[9, 16-17].
CS is a natural polysaccharide obtained from seafood surpluses like shrimps, lobster, and crab shells. It possesses great physiochemical characteristics and has been applicable in extensive fields of research such as water purification, thermal insulation, and biomedical applications[18-21]. CS can form gels easily based on its active functional groups in the aqueous form[22]. Due to such characteristics, it is used in various areas such as catalysts and drug delivery[23-28].
Aerogels as an ultra-porous material with the lowest density, large surface area, and mechanically robustness have been famous in recent years with plenty of applications. Composite aerogels based on nanotubes and graphene are used for thermal insulation[29-30]. However, the manufacturing of such aerogels is costly and complex, which restricts their practical usage. Therefore, researches are needed for fabricating cost-effective and low thermal insulated aerogels[31-32]. Many cellulose-based composite aerogels have already been reported for thermal insulation applications[33-34].
Directional freezing affects the resulted characteristics of aerogels due to microstructure variation in anisotropic aerogels. Such anisotropic aerogels have been mentioned in Refs. [35-40]. Therefore, preparing nano-fibrillated aerogels with excellent performance is still a challenge. CS possesses superior biocompatibility and intrinsic antibacterial characteristics[41-43].
Herein, a novel approach is ued to fabricate CNF/CS anisotropic aerogels employing random and unidirectional freezing. The structural behavior and its effect on resultant composite aerogels properties are observed. CS acts as a structural robustness material and provides mesoporosity within the geometry, whereas cellulose provides supportive bridges within the layers and results in good formability.
1 Experiments
1.1 Materials
CNFs were provided by Liontec Biopharma Co., Ltd., Tianjin, China. CS was bought from Titan Technology Co., Ltd., Shanghai, China. Formaldehyde was purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Acetic acid and sodium hydroxide were provided by Aladdin Reagent Co., Ltd., Shanghai, China. All chemicals were analytically pure.
1.2 Preparation of CNF/CS hydrogel
The CNF solution has been prepared by the addition of deionized water into suspension, which is dispersed at 11 000 r/min using a high-speed homogenizer for 45 min. Meanwhile, CS solutions have been prepared by mixing CS in CNF suspension and glacial acetic acid solution with subsequent magnetic stirring in 80 ℃ heated oil bath for 40 min to get a translucent and uniform suspension. CNF/CS suspensions were further mixed for an additional 30 min to obtain a viscous suspension.
1.3 Fabrication of aerogels
The CNF/CS hydrogel was poured into a flat surface mold and directly placed into a liquid nitrogen bath for freezing, following a lyophilizing process for 48 h at -50 ℃ using a freeze dryer at a pressure of 1 Pa. The prepared aerogels were generally named as random-CNF/CS (r-CNF/CS) aerogels.
The unidirectional freeze-dried aerogels were prepared by transferring suspension into the polytetrafluoroethylene (PTFE) mold while being placed on a copper square bar in the liquid nitrogen-filled bath for 20 min and lyophilized for 60 h. The prepared aerogels were named as unidirectional CNF/CS (u-CNF/CS) aerogels.
2 Characterization
2.1 Morphology observation
The microscopic architecture of CNF/CS-based aerogels was observed by using scanning electron microscopy (SEM) (Hitachi S-4800, Japan).
2.2 Fourier transform infrared (FTIR) spectroscopy
The chemical structures of CNF, CS, and CNF/CS aerogels were observed by conducting FTIR testing with spectra range from 4 000 cm-1to 400 cm-1in the transmission mode at the resolution of 4 cm-1.
2.3 Thermogravimetric analysis (TGA)
Thermal stability of aerogels was studied by a thermogravimetric analyzer (PerkinElmer Inc., USA) at a nitrogen flow rate of 20 mL/min, a temperature range from 30 ℃ to 600 ℃, and a heating rate of 10 ℃/min.
2.4 Thermal conductivity
Thermal conductivityλwas obtained by using a hot disk thermal analyzer (TPS-2500S, Hot Disk AB Co., Sweden) following the transient plane source (TPS) method.
2.5 Compression testing
The compression experiments were conducted by using an Instron 5944 universal machine (Instron,USA) with 500 N load cells at a strain rate of 2 mm/min and a strain of 60%.
3 Results and Discussion
3.1 Structural characterization
The crosslinking and hydrogel formation mechanism of CNF/CS aerogels is shown in Fig.1. The random aerogels were prepared by pouring CNF/CS hydrogel in the flat surface plastic mold, and directly frozen in liquid nitrogen as shown in Fig. 1(b). Whereas for u-CNF/CS aerogel, the hydrogel was transferred into a PTFE mold by putting on the iron mold, and frozen. The resultant aerogels contain entirely different micro-structures as shown in Fig.1(c).
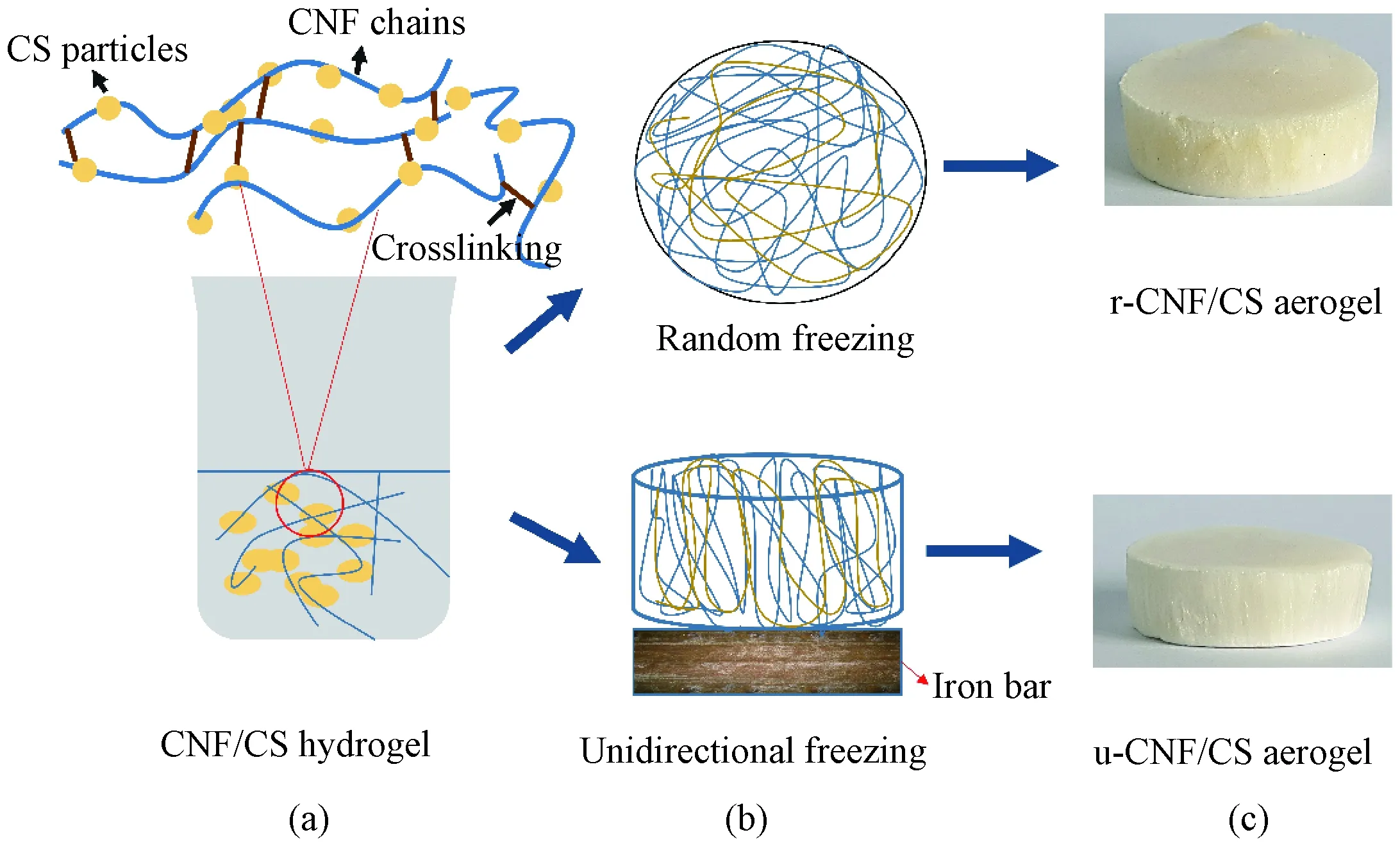
Fig. 1 Mechanism of r-CNF/CS and u-CNF/CS aerogels formation: (a) CNF/CS hydrogel; (b) random and unidirectional freezings; (c) r-CNF/CS and u-CNF/CS aerogels
The structural morphology of CNF/CS aerogels has been observed by using SEM. It can be seen in Figs. 2(a) and 2(b) that the r-CNF/CS aerogels show similar morphology along axial and radial directions because of random temperature gradient during liquid nitrogen freezing and random size of pores forming within the geometry of aerogels. This structure shows the isotropic behavior along both orientations. However, in u-CNF/CS aerogels, the structural orientation is different along the axial and radial directions as shown in Figs. 2(c) and 2(d). This anisotropic morphology of aerogels is formed because of different temperature gradients along both directions. This structure is suitable for the thermal conductivity of aerogels. Along the radial direction, the passage of heat is slow because of the Knudsen effect, while the thermal conductivity within axial geometry of u-CNF/CS aerogels is much faster because of a favorable lamellar structure.
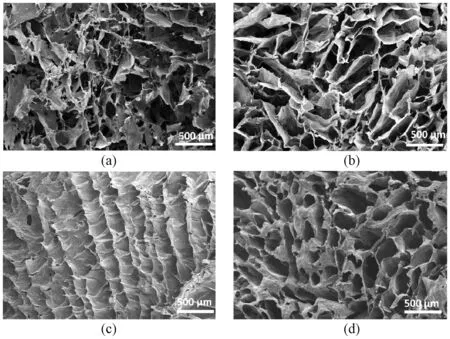
Fig. 2 Schematic illustration of CNF/CS aerogels: (a) axial view of r-CNF/CS aerogels; (b) radial view of r-CNF/CS aerogels; (c) axial view of u-CNF/CS aerogels; (d) radial view of u-CNF/CS aerogels
The addition of CS to the assembly of CNF controls the microstructure alignment and lets the layers be formed in anisotropic aerogels. This microstructure then becomes favorable for heat insulation[31]. CNFs provide fibrous support to the porous assembly, and CS particles add strength to the structure by forming linking bonds within the geometry. With the addition of CS particles to the CNF hydrogel, the structure is changed significantly.
3.2 FTIR spectra of CNF/CS aerogels
Figure 3 shows the FTIR spectra of CNF, CS, and CNF/CS aerogels, respectively. CS particles are doped into cellulose with formaldehyde crosslinking, and resulted in characteristic peaks. CNF and CS share the common functional groups because they are both polysaccharides. The stretching vibrations at 3 200 cm-1and 2 893 cm-1conform to —OH and C—H bonds, respectively.The peak at about 3 200 cm-1is stretching vibrations of —OH and —NH. The peak of CS aerogels at 1 500 cm-1is assigned to the amide group. However, in composite aerogels, the new peak at 1 400 cm-1is formed after crosslinking reaction which corresponds to the amide bond formation between formaldehyde and CS. In composite aerogels, the sharp peak at about 1 600 cm-1is evidence of cross-linked aerogels which corresponds to aldehyde group formation. Formaldehyde addition to aerogels develops structural stability and enhances the mechanical strength of final aerogels by providing strengthening units within the micro-orientation of aerogels.
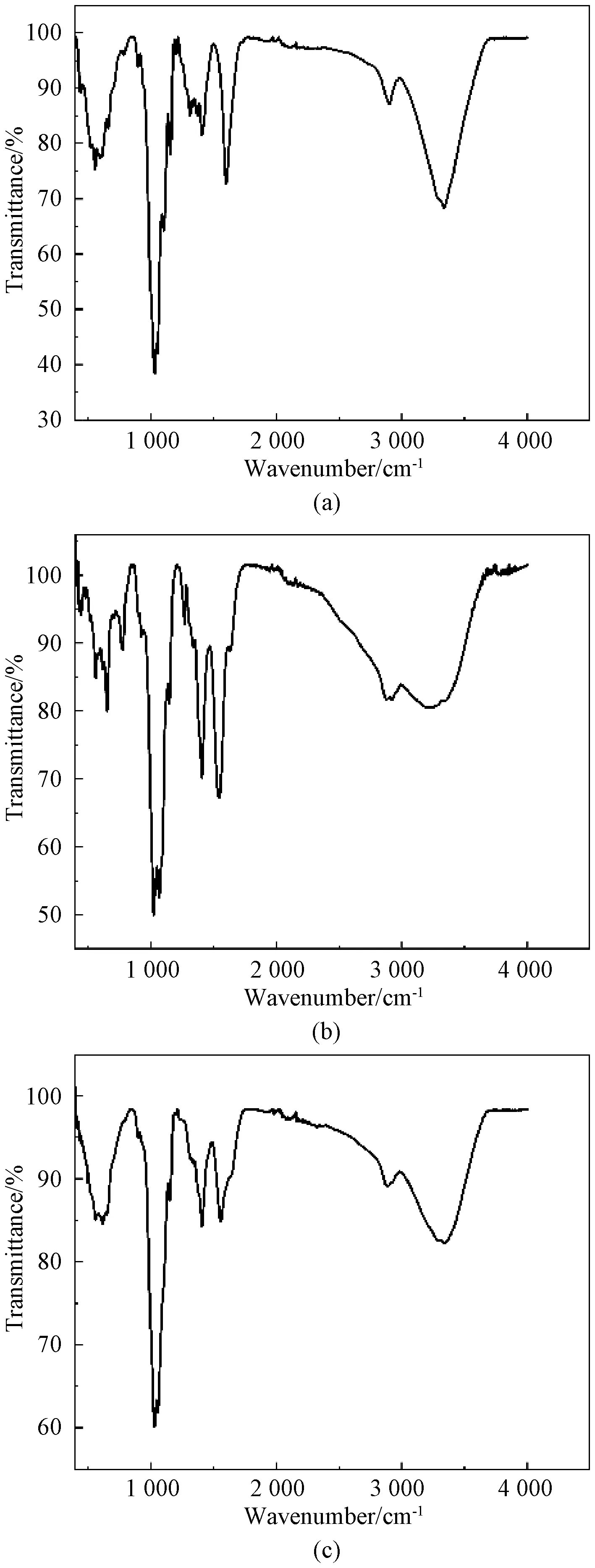
Fig. 3 FTIR spectra: (a) CNF aerogels; (b) CS aerogels; (c) CNF/CS aerogels
3.3 Thermal stability of composite aerogels
The thermal stability of CNF/CS aerogels has been observed from TGA as shown in Fig. 4. The TGA curve of CNF/CS aerogels shows that the weight loss rate is reduced to 90% compared to that of pure CNF and CS aerogels, and the mass retention percentage also improves to 80% in composite aerogels (Fig. 4(b)).

Fig. 4 Thermal behavior of CS, CNF and CNF/CS aerogels: (a)TGA curve; (b) derivative thermogravimetry (DTG) curve
The addition of CS to CNF enhances the thermal stability of CNF/CS aerogels and gives a more stable structure by adding bridges and nanopores to the structure. The microstructure of final aerogels contains nano-sheets that restrain the CNF/CS aerogels from combustion at low temperatures of about 275 ℃ by enhancing its glass transition temperatureTgto 283 ℃. As it can be seen in the endothermic histogram of CNF/CS aerogels, the peak increases significantly which shows the increase ofTgof final aerogels.
The thermal conductivity values of aerogels were collected by using a hot disk apparatus. It was observed that CNF (0.034 W/(m ·K)) and CS (0.037 W/(m ·K)) with their less thermal conductivityλcombined in aerogel form, and resulted with the overall lowestλof composite aerogels (Fig. 5). The u-CNF/CS aerogels contain lowerλ(0.030 W/(m ·K)) as compared to r-CNF/CS aerogels (0.036 W/(m ·K)), because of micro-porous structural variation. The random pore orientation in randomly frozen aerogels results in a faster flow of heat through the assembly due to the fast heat conduction rate, as compared to the aligned lamellar structure of unidirectional frozen aerogels. The lamella in u-CNF/CS aerogels blocks heat conduction to pass through the geometry which results in the lowest thermal conductivity of the specific aerogels. It shows the thermal behavior of aerogels which makes CNF/CS aerogels promising in thermal insulation and flame retardancy applications.
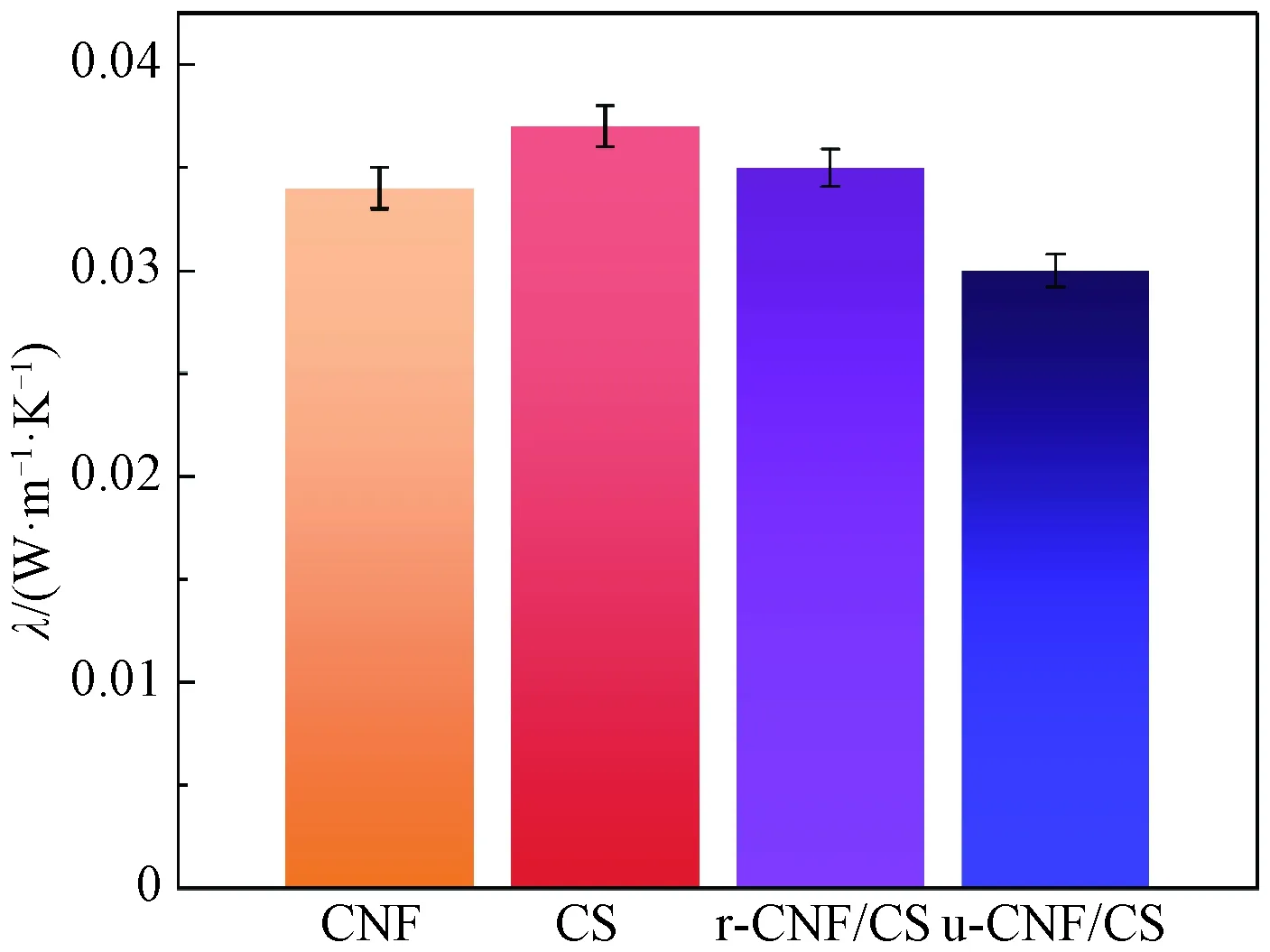
Fig. 5 Thermal conductivity of CNF, CS, r-CNF/CS and u-CNF/CS aerogels
3.4 Mechanical properties of CNF/CS aerogels
The stress-strain curves of CNF, CS and CNF/CS aerogels were collected at a strain of 60% as shown in Fig. 6. The stress-strain behavior of aerogels consists of three stages: the elastic stage at a low strain ratio, yield point, and final densification region. For r-CNF/CS aerogels as shown in Fig. 6(c), the curve is non-linear in the elastic region. However, there is an apparent linear regime in u-CNF/CS as shown in Fig. 6(d). When the stress is released, the strain reaches zero gradually, and the hysteresis loop area of unidirectional composite aerogels gets less relative to pure aerogels as shown in Figs. 6(a) and 6(b). The fast recovery rate of u-CNF/CS aerogels specifies their compressive strength and usability in stress sensors, shape memory alloys,etc.
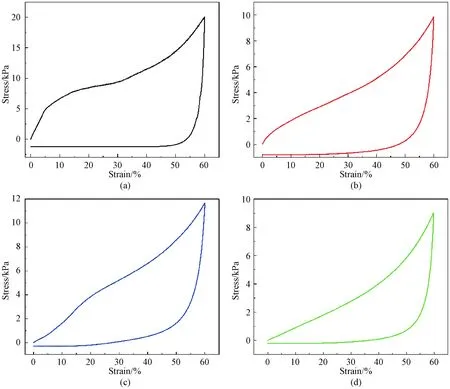
Fig. 6 Stress-strain curves: (a) CNF aerogels; (b) CS aerogels; (c) r-CNF/CS aerogels; (d) u-CNF/CS aerogels
4 Conclusions
In summary, the CNF/CS aerogels have been successfully fabricated by employing two different freeze-drying techniques: random freezing and unidirectional freezing. Aerogels behave differently on different microstructures. CS addition to CNF hydrogel is able to form the thermally resistant and mechanically robust aerogels. FTIR spectra show that CS can form hydrogen bonds with cellulose on the addition of formaldehyde, and reduce bending vibrations of —OH. TGA shows good thermal stability of CNF/CS aerogels because of strong intermolecular forces, andTgof CNF/CS aerogels increases to 283 ℃. The thermal conductivity test shows that the u-CNF/CS aerogels exhibit ultra-low thermal conductivity of around 0.030 W/(m ·K) compared to r-CNF/CS aerogels withλof 0.036 W/(m ·K). Whereas, the stress-strain curves display that u-CNF/CS aerogels are compressible because of the layered structure, and able to regain their original position on the removal of strain. The temperature stability, low thermal conductivity, and better mechanical strength make u-CNF/CS aerogels a safe choice for heat-resistant applications such as automobiles, aerospace, building, extreme environmental conditions, stress sensors, and shape memory alloys.
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Electrospinning of Bead-on-String Sodium Alginate Nanofibrous Membrane
- Eco-Friendly pH Indicator Based on Natural Anthocyanins from Lycium ruthenicum
- Formulating Novel Halogen-Free Synergistic Flame Retardant Epoxy Resins for Vacuum Assisted Resin Infusion Composites
- Electrochemical Reduction Determination of N-Nitrosodiphenylamine in Food Based on Graphene Electrode Material
- Hydrothermal Synthesis of Ordered ZnO Nanorod Arrays by Nanosphere Lithography Method
- Temperature-Dependent Growth of Ordered ZnO Nanorod Arrays
