5种直接抗病毒药物治疗慢性丙型病毒性肝炎有效性与安全性比较的Meta分析
2021-07-11金敏陈平钰李洪超马爱霞
金敏 陈平钰 李洪超 马爱霞


中图分类号 R978.7 文献标志碼 A 文章编号 1001-0408(2021)10-1262-10
DOI 10.6039/j.issn.1001-0408.2021.10.18
摘 要 目的:比较格卡瑞韦(GLE)/哌仑他韦(PIB)、来迪派韦(LDV)/索磷布韦(SOF)、SOF/维帕他韦(VEL)、艾尔巴韦(EBR)/格拉瑞韦(GZR)复合制剂和达诺瑞韦(DNV)+聚乙二醇干扰素联合利巴韦林(P/R)等5种直接抗病毒药物方案治疗慢性丙型病毒性肝炎的有效性与安全性。方法:计算机检索PubMed、Embase、Cochrane图书馆、Web of Science、中国知网、维普网、万方数据等数据库,检索时间均为建库起至2020年6月,收集5种直接抗病毒药物方案治疗慢性丙型病毒性肝炎的随机对照试验(RCT)。筛选文献、提取数据后,采用Cochrane系统评价员手册5.1.0推荐的偏倚风险评估工具对纳入文献质量进行评价,采用Stata 15.0软件进行Meta分析。结果:共纳入48项RCT,试验组患者共计12 227例。Meta分析结果显示,获得持续病毒学应答(SVR)率由高到低依次为GLE/PIB>LDV/SOF>SOF/VEL>EBR/GZR>DNV+P/R,其中GLE/PIB、LDV/SOF、SOF/VEL、EBR/GZR的加权SVR率均在95%以上。任何严重的不良事件发生率、任何不良事件发生率由低到高依次均为EBR/GZR 关键词 慢性丙型病毒性肝炎;直接抗病毒药物;有效性;安全性;Meta分析 Meta-analysis of Efficacy and Safety of 5 Direct Antiviral Agents in the Treatment of Chronic Hepatitis C Infection JIN Min1,CHEN Pingyu1,2,LI Hongchao1,2,MA Aixia1,2(1. School of International Pharmaceutical Business, China Pharmaceutical University, Nanjing 211198, China; 2. Center for Pharmacoeconomics and Outcomes Research, China Pharmaceutical University, Nanjing 211198, China) ABSTRACT OBJECTIVE: To compare the efficacy and safety of 5 direct antiviral agents in the treatment of chronic hepatitis C infection as glecaprevir (GLE)/pibrentasvir (PIB), ledipasvir (LDV)/sofosbuvir (SOF), SOF/velpatasvir (VEL), elbasvir (EBR)/grazoprevir (GZR) compound preparation and danoprevir (DNV)+peginterferon combined with ribavirin (P/R). METHODS: Retrieved from PubMed, Embase, Cochrane Library, Web of Science, CNKI, VIP, Wanfang database and other databases, RCTs about 5 direct antiviral agents in the treatment of chronic hepatitis C infection were collected during the inception to Jun. 2020. After literature screening and data extraction, the quality of included literatures were evaluated with bias risk evaluation tool recommended by Cochrane system evaluator manual 5.1.0. Meta-analysis was performed by using Stata 15.0 software. RESULTS: A total of 48 RCTs with 12 227 patients in trial group were included. Results of Meta-analysis showed that the descending order of sustained virological response (SVR) rate was GLE/PIB>LDV/SOF>SOF/VEL>EBR/GZR>DNV+P/R; weighted SVR rates of GLE/PIB, LDV/SOF, SOF/VEL and EBR/GZ were more than 95%. The incidence of any severe adverse event and adverse event in ascending order was EBR/GZR
KEYWORDS Chronic hepatitis C infection; Direct antiviral agent; Efficacy; Safety; Meta-analysis
丙型病毒性肝炎是由丙型肝炎病毒(Hepatitis C virus,HCV)感染引起的传染病,普通人群感染HCV后可能发展为慢性丙型病毒性肝炎(以下简称“慢性丙肝”)[1]。2015年,全球约有7 100万人感染HCV,且每年的新增病例约300万[2]。HCV可分为6种基因分型,我国的慢性丙肝类型包含HCV1、HCV2、HCV3和HCV6型,且以HCV1b型感染居多[3-4]。基于慢性丙肝引起的不良后果及其传染性对公共卫生的巨大威胁,同时基于直接抗病毒药物(DAAs)显著的疗效,世界卫生组织(WHO)提出了“2030丙肝消除计划”,旨在实现2030年消除病毒性肝炎對公共卫生威胁这一目标[1,5]。慢性丙肝的抗病毒治疗以患者获得持续病毒学应答(SVR)为目标,且经治疗后获得SVR的患者视为达到病毒学治愈的标准[1]。
传统的慢性丙肝治疗方案为聚乙二醇干扰素联合利巴韦林(以下简称“P/R”),虽然该方案的价格较低,但患者的SVR率也较低[6]。2010年后,安全高效的DAAs成为慢性丙肝的主要推荐治疗方案,主要包括来迪派韦(LDV)/索磷布韦(SOF)、SOF/维帕他韦(VEL)、格卡瑞韦(GLE)/哌仑他韦(PIB)、艾尔巴韦(EBR)/格拉瑞韦(GZR)的复合制剂以及达诺瑞韦(DNV)联合P/R方案[1]。同时,上述药物也是我国医保支付重点关注的药物。这些DAAs主要靶向HCV的非结构蛋白,抑制HCV RNA的转录,从而发挥治疗HCV感染的作用[7]。根据作用靶蛋白的不同,DAAs分为NS3/4A蛋白酶抑制剂(如GLE、GZR)、NS5B抑制剂(如SOF)和NS5A抑制剂(如PIB、LDV、VEL、EBR、DNV)[7]。经DAAs治疗后,患者的SVR率较传统P/R方案有所提高,同时药物相互作用和不良事件也较少[1]。目前,国内外已有关于DAAs治疗慢性丙肝的研究,但这些研究多数为单一用药方案,且结论尚未统一[8-55]。同时,由于DAAs药物众多、疗效接近,尚无针对多种DAAs治疗不同基因型、治疗史及肝硬化状态的综合评价。基于此,本研究采用Meta分析的方法比较了GLE/PIB、LDV/SOF、SOF/VEL、EBR/GZR复合制剂和DNV+P/R等5种直接抗病毒药物方案治疗慢性丙肝的有效性与安全性,旨在为临床用药提供循证参考。
1 资料与方法
1.1 纳入与排除标准
根据PICOS(P表示研究对象,I表示干预措施,C表示对照措施,O表示干预措施的诊疗效果,S表示研究设计方案)原则[56]设定本研究文献的纳入与排除标准。
1.1.1 研究类型 国内外公开发表的随机对照试验(RCT);语种限定为中文和英文。
1.1.2 研究对象 年龄≥18岁;HCV感染超过 6 个月或感染日期不明;抗HCV 及 HCV RNA阳性,即HCV RNA载量≥1×104 IU/mL,肝脏组织病理学检查符合《丙型肝炎防治指南(2019年版)》中的相关诊断标准[1];HCV基因型和肝硬化状态不限。
1.1.3 干预措施 试验组以GLE/PIB、LDV/SOF、SOF/VEL、EBR/GZR复合制剂和DNV+P/R方案等为干预措施,剂量和用法用量不限。本研究未具体限定对照组的干预措施,其措施包括安慰剂、延迟治疗或其他等。
1.1.4 结局指标 有效性指标:①SVR率。安全性指标:②任何严重的不良事件,③任何不良事件以及经调研后认为需要处理的不良反应(包括④恶心/呕吐、⑤皮疹、⑥失眠)。SVR率=SVR的患者例数/总例数×100%[1]。
1.1.5 排除标准 ①未报告所需结局指标的文献;②病例报告和观察性研究;③摘要;④综述;⑤描述性报告和述评;⑥重复发表的文献;⑦会议论文。
1.2 文献检索策略
计算机检索PubMed、Embase、Cochrane图书馆、Web of Science、中国知网、维普网、万方数据等数据库。英文检索词为“HCV”“Hepatitis C”“Ledipasvir”“Sofosbuvir”“Velpatasvir”“Glecaprevir”“Pibrentasvir”“Elbasvir”“Grazoprevir”“Danoprevir”;中文检索词为“丙型肝炎”“丙肝”“来迪派韦”“索磷布韦”“维帕他韦”“格卡瑞韦” “哌仑他韦”“艾尔巴韦”“格拉瑞韦”“达诺瑞韦”等,采用主题词与检索词结合的检索方式。检索时限均为各数据库建库起至2020年6月。同时向各药企咨询,由药企医学部提供其他途径的补充文献。
1.3 文献筛选与资料提取
由两名研究者根据纳入与排除标准独立筛选文献、提取资料并交叉核对;如遇分歧,则由第3名研究者协助判断。根据事先设计好的数据提取表格提取相关信息,包括第一作者及发表年份、例数、性别、年龄、干预措施和结局指标等。
1.4 文献质量评价
采用Cochrane系统评价员手册5.1.0推荐的偏倚风险评估工具对纳入文献质量进行评价,包括随机方法、分配隐藏、对受试者和研究者施盲、结局评估的盲法、结果数据完整性、选择性报告结果和其他偏倚来源,每个方面均分为低偏倚风险、不清楚和高偏倚风险[57]。
1.5 统计学方法
采用Stata 15.0软件进行Meta分析。对于不同干预措施的有效性指标,计算其合并的加权SVR率、加权不良事件发生率和相应的效应量(ES)及95%置信区间(CI);计数资料采用相对危险度(RR)及其95%CI表示;计量资料则采用加权均数差(WMD)及其95%CI表示;连续型变量的结局指标采用WMD进行统计合并。各研究间异质性采用χ2检验和I 2检验,若各研究间无统计学异质性(P>0.1,I 2≤50%),采用固定效应模型分析;反之,则采用随机效应模型分析。采用倒漏斗图进行发表偏倚分析。P<0.05为差异有统计学意义。
2 结果
2.1 文献检索结果与纳入研究基本信息
初检各数据库共获得相关文献8 465篇,其他途径获得文献4篇。经阅读标题、摘要及全文后,最终纳入文献48篇[8-55],试验组患者共计12 227例。其中,GLE/PIB有8篇[11,15,27,45-46,51,53-54]、LDV/SOF有15篇[8-10,14,24,26,31-32,37,39-40,42-43,47,50]、SOF/VEL有9篇[12,16,18-20,22,29,43-44]、EBR/GZR有12篇[13,17,23,28,34-36,38,48-49,52,55]、DNV+P/R有5篇[21,25,30,33,41]。文献筛选流程见图1;纳入研究基本信息(试验组)见表1(表中,除明确说明用药频次外,其余用药频次均为每天1次;RBV表示利巴韦林,若无特殊说明,其剂量为按患者体质量给药,即<75 kg者每天1 000 mg,≥75 kg者每天1 200 mg;RTV表示利托那韦;GLE/PIB、LDV/SOF、SOF/VEL、EBR/GZR、RTV均为片剂,口服给药;P/R为注射剂,静脉注射给药,由于本研究对照组的干预措施未作具体限定,故表中未列出对照组信息)。
2.2 纳入文献质量评价结果
36项研究提及随机序列产生的方法[9-10,12,14-15,17,19,21,23-29,31-42,44-46,48-52,54],19项研究采用分配隐藏[12-13,17,20-21,23,25-26,28,33,35,37,39,41,44,48-49,52,54];7项研究使用盲法[11-12,20,28,34,52,54];40項研究结果数据完整[8-10,12-30,32-35,37,39,41,44-49,51-55];所有研究均未选择性报告,均不清楚是否存在其他偏倚来源,详见图2、图3。
2.3 Meta分析结果
2.3.1 SVR率 GLE/PIB等5种药物方案治疗SVR率的Meta分析结果如表2所示。
①GLE/PIB——有8项研究报道了GLE/PIB治疗的SVR率[11,15,27,45-46,51,53-54]。Meta分析结果显示,GLE/PIB治疗的加权SVR率为99%[95%CI(0.98,0.99),P<0.001]。
②LDV/SOF——有15项研究报道了LDV/SOF 治疗的SVR率[8-10,14,24,26,31-32,37,39-40,42-43,47,50]。Meta分析结果显示,LDV/SOF治疗的加权SVR率为97%[95%CI(0.96,0.97),P<0.001]。
③SOF/VEL——有9项研究报道了SOF/VEL治疗的SVR率[12,16,18-20,22,29,43-44]。Meta分析结果显示,SOF/VEL治疗的加权SVR率为96%[95%CI(0.95,0.97),P<0.001]。
④EBR/GZR——有12项研究报道了EBR/GZR 治疗的SVR率[13,17,23,28,34-36,38,48-49,52,55]。Meta分析结果显示,EBR/GZR治疗的加权SVR率为95%[95%CI(0.93,0.96),P<0.001]。
⑤DNV+P/R——有5项研究报道了DNV+P/R治疗的SVR率[21,25,30,33,41]。Meta分析结果显示,DNV+P/R治疗的加权SVR率为69%[95%CI(0.60,0.77),P<0.001]。
亚组分析结果如表3所示(表中P值均小于0.05)。
①HCV基因型的不同——分别有25项[8-9,12,14,19,21,25-27,29-34,36-39,41-42,44-45,49,54]、8项[10-11,13,19,22,29,42,51]、11项[18-19,22-24,27,38,42,44,53-54]、4项[40,42,47,50]和3项[15,24,43]研究报道了HCV 1、2、3、4、6型患者的SVR率。结果,GLE/PIB治疗HCV 1、2型的加权SVR率较高,分别为100%[95%CI(0.99,1.00),P<0.05]、99%[95%CI(0.98,1.00),P<0.05];EBR/GZR治疗HCV 3型的加权SVR率较高,为99%[95%CI(0.98,1.00),P<0.05];SOF/VEL治疗HCV 6型的加权SVR率较高,为100%[95%CI(0.99,1.00),P<0.05]。
②肝硬化——分别有16项[12,14-16,18,26-27,30,34,36,38-40,44,50,54]、23项[11-15,19,21,25-26,30,32,34,37-38,40-41,43-45,49-51,53]研究报道了伴或不伴肝硬化患者的SVR率。结果,对于伴或不伴肝硬化的患者,GLE/PIB治疗的加权SVR率均较高,分别为100%[95%CI(0.99,1.00),P<0.05]、99%[95%CI(0.98,1.00),P<0.05]。
③治疗史——分别有22项[9,13,17,19,23-26,30,32,35-38,41-43,47-49,52-53]、24项[8,12-13,15,17,21,23-24,26-27,29,31,34-37,42,44-47,50-51,53]研究报道了初治和经治患者的SVR率。结果,GLE/PIB初治患者的加权SVR率较高,为99%[95%CI(0.98,1.00),P<0.05];LDV/SOF经治患者的加权SVR率较高,为99%[95%CI(0.98,1.00),P<0.05]。
④ 疗程——分别有1项[26]、9项[15,19,32,37,39,43,47,51,54]、28项[8-12,14-16,18-20,22,24,26-27,29,31,37,40,42-47,50,53-54]、2项[46,53]和7项[8-9,14,16,29,40,50]研究报道了治疗6、8、12、16、24周的SVR率。结果,当疗程为8周和12周时,均以GLE/PIB治疗的加权SVR率较高,分别为98%[95%CI(0.97,0.99),P<0.05]、99%[95%CI(0.98,1.00),P<0.05];当疗程为24周时,LDV/SOF治疗的加权SVR率较高,为98%[95%CI(0.97,0.99),P<0.05]。
⑤联合用药——分別有25项[8-9,13-14,16-19,23-24,26-27,29,32,35,37-40,42,44-45,47,49-50]、37项[8-13,15-16,18-20,22-24,26-28,31-32,34-35,38-39,42-55] 、2项[17,36]、2项[17,23]研究报道了联合RBV或不联合RBV、联合SOF或不联合SOF治疗的SVR率。结果,LDV/SOF联合RBV治疗的加权SVR率,为98%[95%CI(0.97,0.99),P<0.05],高于LDV/SOF不联合RBV治疗的加权SVR率93%[95%CI(0.91,0.96),P<0.05];SOF/VEL不联合RBV治疗的加权SVR率为97%[95%CI(0.96,0.98),P<0.05],高于SOF/VEL联合RBV治疗的加权SVR率94%[95%CI(0.91,0.97),P<0.05];EBR/GZR不联合RBV治疗的加权SVR率为96%[95%CI(0.94,0.97),P<0.05],高于EBR/GZR联合RBV治疗的加权SVR率91%[95%CI(0.88,0.95),P<0.05]。
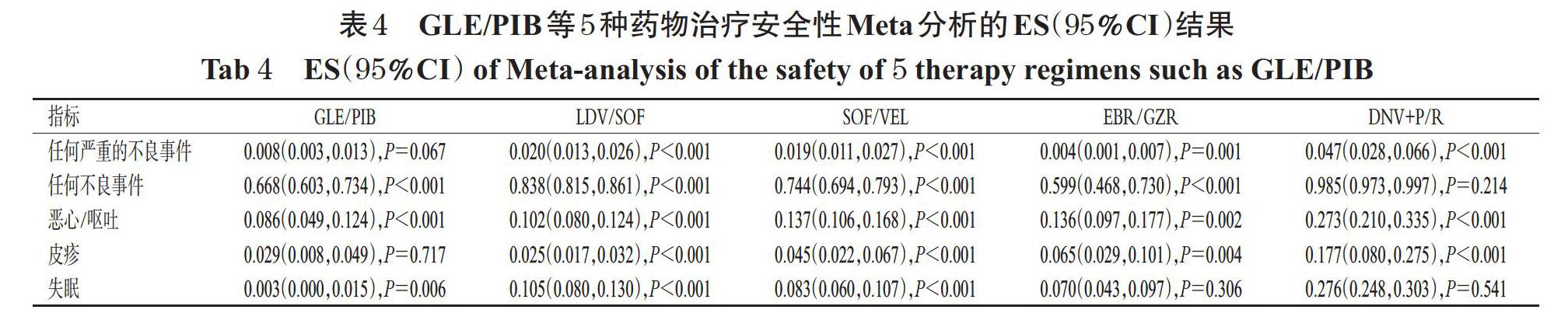
2.3.2 安全性 分别有46项[8-30,32-41,43-55]、43项[8-16,18-20,22-30,32-40,43-55]、29项[8-9,11-13,16,19-27,29,32-33,35,37,39,41,43-45,49-51,54]、22项[8-9,12,16,18-22,24,26-27,30,32-33,35,37,40-41,44-45,50]、18项[8-9,15,19,23-26,29,32-33,35,37,40-41,44,50-51]研究报道了患者任何严重的不良事件、任何不良事件、恶心/呕吐、皮疹、失眠发生率。任何严重的不良事件和任何不良事件发生率从低到高依次均为EBR/GZR 2.4 发表偏倚分析 以GLE/PIB治疗的SVR率为指标绘制倒漏斗图,结果见图4。由图4可知,有3个研究散点在倒漏斗图外,其余各研究散点均分布于倒漏斗图范围内,且倒漏斗图两侧分布不对称,提示本研究存在发表偏倚的可能性较大(其余指标所得结果相似,图略)。 3 讨论 直接抗病毒药物是治疗HCV的靶向特异性小分子化合物[58]。近年来,随着抗HCV感染DAAs的出现,慢性丙肝治疗的新时代也随之开启。但DAAs在我国上市时间严重晚于他国,且价格普遍较昂贵,患者因无法负担而导致病情恶化,这使得我国慢性丙肝患者的治疗率较低,给我国乃至全球“丙肝消除计划”的实现造成了阻碍[59]。在真实世界的临床实践中,本研究纳入的5种慢性丙肝治疗方案的疗效显著[60-64]。目前,我国用于治疗慢性丙肝的药物较多,但缺乏DAAs治疗方案之间的比较研究,加之DAAs对于不同基因型、肝硬化状态、治疗史患者的有效性也存在未知性。为此,本研究对5种DAAs治疗慢性丙肝的有效性与安全性进行比较。 本研究结果显示,5种药物方案治疗慢性丙肝的加权SVR率由高到低为GLE/PIB>LDV/SOF>SOF/VEL> EBR/GZR>DNV+ P/R。亚组分析结果显示,对于HCV 1、2型患者,以GLE/PIB治疗的SVR率较高,HCV 3、6型患者分别以EBR/GZR、SOF/VEL治疗的SVR率较高;无论是否伴有肝硬化,均以GLE/PIB治疗的SVR率较高;对于初治患者,GLE/PIB的SVR率较高,而对于经治患者,则LDV/SOF的SVR率较高;疗程方面,8周和12周疗法均以GLE/PIB治疗的SVR率较高;联合和不联合RBV时,分别以LDV/SOF和SOF/VEL治疗的SVR率较高。本研究发现,DNV+P/R治疗的SVR率均显著低于其他4种药物方案,笔者分析其原因可能为DNV为我国首个研发的小分子直接抗病毒药物[65],目前的RCT较少,且通常需要联合P/R或其他DAAs使用。 安全性方面,任何严重的不良事件和任何不良事件发生率从低到高依次均为EBR/GZR 综上所述,GLE/PIB、LDV/SOF、SOF/VEL、EBR/GZR治疗慢性丙肝的有效率较高且接近,尤以GLE/PIB治疗的加权SVR率最佳;安全性方面,以EBR/GZR、GLE/PIB相对较好。本研究的局限性如下:(1)纳入研究的总体质量不高,且多数RCT未实施盲法,具有较高的偏倚风险,可能影响分析结果;(2)由于本研究纳入的RCT在设计上多为两种DAAs直接比较或者同种DAAs不同剂量或不同治疗周期的比较,缺乏5种DAAs方案的直接比较,也缺乏空白对照,因此只进行了单臂Meta分析,而无法进行成组Meta分析或者网络Meta分析,其不确定性较高;(3)纳入的研究大多为国外研究,国内研究较少,在患者基线特征上可能存在差异。因此,本结论尚需更多高质量RCT进一步验证。 参考文献 [ 1 ] 中华医学会肝病学分会,中华医学会感染病学分会.丙型肝炎防治指南:2019年版[J].中华肝脏病杂志,2019,27(12):962-963. [ 2 ] World Health Organization. Global hepatitis report:2017
[R/OL]. [2021-04-15]. https://www.who.int/hepatitis/pub- lications/global-hepatitis-report7.
[ 3 ] RAO H,WEI L,LOPEZ-TALAVERA J C,et al. Distribution and clinical correlates of viral and host genotypes in Chinese patients with chronic hepatitis C virus infection[J]. J Gastroenterol Hepatol,2014,29(3):545-553.
[ 4 ] CHEN Y,YU C,YIN X,et al. Hepatitis C virus genotypes and subtypes circulating in mainland China[J]. Emerg Microbes Infect,2017,6(11):e95.
[ 5 ] World Health Organization. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection:2018[EB/OL]. [2021-04-15]. https://apps.who.int/iris/handle/10665/273174.
[ 6 ] PECORARO V,BANZI R,CARIANI E,et al. New direc- tacting antivirals for the treatment of patients with hepatitis C virus infection:a systematic review of randomized controlled trials[J]. J Clin Exp Hepatol,2019,9(4):522-538.
[ 7 ] 溫晓玉,牛俊奇.直接抗病毒药物治疗慢性丙型肝炎的作用机制[J].临床肝胆病杂志,2016,32(9):1699-1705.
[ 8 ] AFDHAL N,REDDY K R,NELSON D R,et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection[J]. N Engl J Med,2014,370(16):1483-1493.
[ 9 ] AFDHAL N,ZEUZEM S,KWO P,et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection[J]. N Engl J Med,2014,370(20):1889-1898.
[10] ASAHINA Y,ITOH Y,UENO Y,et al. Ledipasvir-sofosbuvir for treating Japanese patients with chronic hepatitis C virus genotype 2 infection[J]. Liver Int,2018,38(9):1552-1561.
[11] ASSELAH T,KOWDLEY K V,ZADEIKIS N,et al. Efficacy of glecaprevir/pibrentasvir for 8 or 12 weeks in patients with hepatitis C virus genotype 2,4,5,or 6 infection without cirrhosis[J]. Clin Gastroenterol Hepatol,2018,16(3):417-426.
[12] BOURLI?RE M,GORDON S C,FLAMM S L,et al. Sofosbuvir,Velpatasvir,and voxilaprevir for previously trea- ted HCV infection[J]. N Engl J Med,2017,376(22):2134-2146.
[13] BROWN A,H?ZODE C,ZUCKERMAN E,et al. Efficacy and safety of 12 weeks of elbasvir±grazoprevir±ribavirin in participants with hepatitis C virus genotype 2,4,5 or 6 infection:the C-SCAPE study[J]. J Viral Hepat,2018,25(5):457-464.
[14] CHARLTON M,EVERSON G T,FLAMM S L,et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease[J]. Gastroenterology,2015,149(3):649-659.
[15] CHAYAMA K,SUZUKI F,KARINO Y,et al. Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 1 hepatitis C virus infection with and without cirrhosis[J]. J Gastroenterol,2018,53(4):557-565.
[16] CURRY M P,OLEARY J G,BZOWEJ N,et al. Sofosbuvir and velpatasvir for HCV in patients with decompensa- ted cirrhosis[J]. N Engl J Med,2015,373(27):2618-2628.
[17] DE L?DINGHEN V,LAFOREST C,H?ZODE C,et al.Retreatment with sofosbuvir plus grazoprevir/elbasvir plus ribavirin of patients with hepatitis C virus genotype 1 or 4 who previously failed an ns5a-or ns3-containing regimen:the anrs HC34 revenge study[J]. Clin Infect Dis,2018,66(7):1013-1018.
[18] ESTEBAN R,PINEDA J A,CALLEJA J L,et al. Efficacy of sofosbuvir and velpatasvir,with and without ribavirin,in patients with hepatitis C virus genotype 3 infection and cirrhosis[J]. Gastroenterology,2018,155(4):1120-1127.
[19] EVERSON G T,TOWNER W J,DAVIS M N,et al. Sofosbuvir with velpatasvir in treatment-naive noncirrhotic patients with genotype 1 to 6 hepatitis C virus infection:a randomized trial[J]. Ann Intern Med,2015,163(11):818- 826.
[20] FELD J J,JACOBSON I M,H?ZODE C,et al. Sofosbuvir and velpatasvir for HCV genotype 1,2,4,5,and 6 infection[J]. N Engl J Med,2015,373(27):2599-2607.
[21] FELD J J,JACOBSON I M,JENSEN D M,et al. Randomized study of danoprevir/ritonavir-based therapy for HCV genotype 1 patients with prior partial or null respon- ses to peginterferon/ribavirin[J]. J Hepatol,2015,62(2):294-302.
[22] FOSTER G R,AFDHAL N,ROBERTS S K,et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection[J]. N Engl J Med,2015,373(27):2608-2617.
[23] FOSTER G R,AGARWAL K,CRAMP M E,et al. Elbasvir/grazoprevir and sofosbuvir for hepatitis C virus genotype 3 infection with compensated cirrhosis:a randomized trial[J]. Hepatology,2018,67(6):2113-2126.
[24] GANE E J,HYLAND R H,AN D,et al. Efficacy of ledipasvir and sofosbuvir,with or without ribavirin,for 12 weeks in patients with HCV genotype 3 or 6 infection[J]. Gastroenterology,2015,149(6):1454-1461.
[25] GANE E J,POCKROS P J,ZEUZEM S,et al. Mericita- bine and ritonavir-boosted danoprevir with or without ribavirin in treatment-naive HCV genotype 1 patients: INFORM-SVR study[J]. Liver Int,2015,35(1):79-89.
[26] GANE E J,STEDMAN C A,HYLAND R H,et al. Efficacy of nucleotide polymerase inhibitor sofosbuvir plus the NS5A inhibitor ledipasvir or the NS5B non-nucleoside inhibitor GS-9669 against HCV genotype 1 infection[J].Gastroenterology,2014,146(3):736-743.
[27] GANE E,POORDAD F,WANG S,et al. High efficacy of ABT-493 and ABT-530 treatment in patients with HCV genotype 1 or 3 infection and compensated cirrhosis[J]. Gastroenterology,2016,151(4):651-659.
[28] GEORGE J,BURNEVICH E,SHEEN I S,et al. Elbasvir/grazoprevir in Asia-Pacific/Russian participants with chro- nic hepatitis C virus genotype 1,4,or 6 infection[J]. Hepatol Commun,2018,2(5):595-606.
[29] IZUMI N,TAKEHARA T,CHAYAMA K,et al. Sofosbuvir-velpatasvir plus ribavirin in Japanese patients with ge- notype 1 or 2 hepatitis C who failed direct-acting antivirals[J]. Hepatol Int,2018,12(4):356-367.
[30] KAO J H,TUNG S Y,LEE Y,et al. Ritonavir-boosted danoprevir plus peginterferon alfa-2a and ribavirin in Asian chronic hepatitis C patients with or without cirrhosis[J]. J Gastroenterol Hepatol,2016,31(10):1757-1765.
[31] KAWAKAMI Y,OCHI H,HAYES C N,et al. Efficacy and safety of ledipasvir/sofosbuvir with ribavirin in chro- nic hepatitis C patients who failed daclatasvir/asunaprevir therapy:pilot study[J]. J Gastroenterol,2018,53(4):548- 556.
[32] KOWDLEY K V,GORDON S C,REDDY K R,et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis[J]. N Engl J Med,2014,370(20):1879-1888.
[33] KOWDLEY K V,LAWITZ E,POORDAD F,et al. Phase 2b trial of interferon-free therapy for hepatitis C virus ge- notype 1[J]. N Engl J Med,2014,370(3):222-232.
[34] KUMADA H,SUZUKI Y,KARINO Y,et al. The combination of elbasvir and grazoprevir for the treatment of chronic HCV infection in Japanese patients:a randomized phase Ⅱ/Ⅲ study[J]. J Gastroenterol,2017,52(4):520- 533.
[35] KWO P,GANE E J,PENG C Y,et al. Effectiveness of Elbasvir and grazoprevir combination,with or without ribavirin,for treatment-experienced patients with chronic he- patitis C infection[J]. Gastroenterology,2017,152(1):164-175.
[36] LAWITZ E,POORDAD F,GUTIERREZ J A,et al.Short- duration treatment with elbasvir/grazoprevir and sofosbuvir for hepatitis C:a randomized trial[J]. Hepatology,2017,65(2):439-450.
[37] LAWITZ E,POORDAD F,HYLAND R H,et al. Ledipasvir/sofosbuvir-based treatment of patients with chronic genotype-1 HCV infection and cirrhosis:results from two phase Ⅱ studies[J]. Antivir Ther,2016,21(8):679-687.
[38] LAWITZ E,GANE E,PEARLMAN B,et al. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir(MK-5172)and elbasvir(MK-8742)with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis(C-WORTHY):a randomised,open-label phase 2 trial[J].Lancet,2015,385(9973):1075-1086.
[39] LAWITZ E,POORDAD F F,PANG P S,et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection(LONESTAR):an open-label,randomised,phase 2 trial[J]. Lancet,2014,383(9916):515-523.
[40] MANNS M,SAMUEL D,GANE E J,et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver di- sease:a multicentre,open-label,randomised,phase 2 trial [J]. Lancet Infect Dis,2016,16(6):685-697.
[41] MARCELLIN P,COOPER C,BALART L,et al. Rando- mized controlled trial of danoprevir plus peginterferon alfa2a and ribavirin in treatment-na?ve patients with hepatitis C virus genotype 1 infection[J]. Gastroenterology,2013,145(4):790-800.
[42] MIZOKAMI M,YOKOSUKA O,TAKEHARA T,et al.Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 he- patitis C:an open-label,randomised,phase 3 trial[J]. Lancet Infect Dis,2015,15(6):645-653.
[43] NGUYEN E,TRINH S,TRINH H,et al. Sustained virologic response rates in patients with chronic hepatitis C genotype 6 treated with ledipasvir+sofosbuvir or sofosbuvir+velpatasvir[J]. Aliment Pharmacol Ther,2019,49(1):99-106.
[44] PIANKO S,FLAMM S L,SHIFFMAN M L,et al. Sofosbuvir plus velpatasvir combination therapy for treatment-experienced patients with genotype 1 or 3 hepatitis C virus infection:a randomized trial[J]. Ann Intern Med,2015,163(11):809-817.
[45] POORDAD F,FELIZARTA F,ASATRYAN A,et al. Glecaprevir and pibrentasvir for 12 weeks for hepatitis C virus genotype 1 infection and prior direct-acting antiviral treatment[J]. Hepatology,2017,66(2):389-397.
[46] POORDAD F,POL S,ASATRYAN A,et al. Glecaprevir/pibrentasvir in patients with hepatitis C virus genotype 1 or 4 and past direct-acting antiviral treatment failure[J].Hepatology,2018,67(4):1253-1260.
[47] SHIHA G,ESMAT G,HASSANY M,et al. Ledipasvir/sofosbuvir with or without ribavirin for 8 or 12 weeks for the treatment of HCV genotype 4 infection:results from a randomised phase Ⅲ study in Egypt[J]. Gut,2019,68(4):721-728.
[48] SPERL J,HORVATH G,HALOTA W,et al. Efficacy and safety of elbasvir/grazoprevir and sofosbuvir/pegylated interferon/ribavirin:a phase Ⅲ randomized controlled trial
[J]. J Hepatol,2016,65(6):1112-1119.
[49] SULKOWSKI M,HEZODE C,GERSTOFT J,et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir(MK-5172)and elbasvir(MK-8742)with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection(C-WORTHY):a randomised,open-label phase 2 trial
[J]. Lancet,2015,385(9973):1087-1097.
[50] TAM E,LUETKEMEYER A F,MANTRY P S,et al. Ledipasvir/sofosbuvir for treatment of hepatitis C virus in sofosbuvir-experienced,NS5A treatment-naive patients:findings from two randomized trials[J]. Liver Int,2018,38(6):1010-1021.
[51] TOYODA H,CHAYAMA K,SUZUKI F,et al. Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 2 hepatitis C virus infection[J].Hepatology,2018,67(2):505-513.
[52] WEI L,JIA J D,WANG F S,et al. Efficacy and safety of elbasvir/grazoprevir in participants with hepatitis C virus genotype 1,4,or 6 infection from the Asia-Pacific region and Russia:final results from the randomized C-CORAL study[J]. J Gastroenterol Hepatol,2019,34(1):12-21.
[53] WYLES D,POORDAD F,WANG S,et al. Glecaprevir/pibrentasvir for hepatitis C virus genotype 3 patients with cirrhosis and/or prior treatment experience:a partially randomized phase 3 clinical trial[J]. Hepatology,2018,67(2):514-523.
[54] ZEUZEM S,FOSTER G R,WANG S,et al. Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection[J]. N Engl J Med,2018,378(4):354-369.
[55] ZEUZEM S,GHALIB R,REDDY K R,et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1,4,or 6 infection:a randomized trial[J].Ann Intern Med,2015,163(1):1-13.
[56] 李雪迎. Meta分析研究設计中的PICOS原则[J].中国介入心脏病学杂志,2016,24(11):611.
[57] HIGGINS J P,ALTMAN D G,G?TZSCHE P C,et al. The Cochrane collaborations tool for assessing risk of bias in randomised trials[J]. BMJ,2011,343:d5928.
[58] 王靖,何小羊.抗丙型肝炎病毒药物研究进展[J].国际药学研究杂志,2015,42(5):551-560.
[59] 孟蕊,芮明军,马越,等.治疗丙肝的第二代直接抗病毒药物的经济性系统评价[J].中国药房,2020,31(23):2882- 2888.
[60] LIU C H,LIU C J,HUNG C C,et al. Glecaprevir/pibrentasvir for patients with chronic hepatitis C virus infection:real-world effectiveness and safety in Taiwan[J]. Liver Int,2020,40(4):758-768.
[61] CHIU W N,HUNG C H,LU S N,et al. Real-world effectiveness of glecaprevir/pibrentasvir and ledipasvir/sofosbuvir for mixed genotype hepatitis C infection:a multicenter pooled analysis in Taiwan[J]. J Viral Hepat,2020,27(9):866-872.
[62] 李剑萍,陈学福,严勤,等.索磷布韦维帕他韦联合或不联合利巴韦林治疗中国成人慢性丙型肝炎病毒感染者的疗效和安全性[J].中华肝脏病杂志,2020,28(10):831- 837.
[63] KRAMER J R,PUENPATOM A,ERICKSON K F,et al. Real-world effectiveness of elbasvir/grazoprevir in HCV- infected patients in the US veterans affairs healthcare system[J]. J Viral Hepat,2018,25(11):1270-1279.
[64] 杨晓冬,贾婷,张秀灵,等.达诺瑞韦联合长效α-干扰素治疗基因3型慢性丙型肝炎患者疗效研究[J].实用肝脏病杂志,2021,24(1):35-38.
[65] 杨臻峥,孙友松,魏利军,等. 2014至2018年我国自主研发并获准上市的1类新药概述[J].中国新药杂志,2019,28(13):1537-1546.
[66] 黄建荣,张文宏.以达诺瑞韦为基础的抗病毒方案在慢性丙型肝炎治疗中的临床研究结果解读[J].中华传染病杂志,2018,36(10):594-598.
(收稿日期:2020-12-08 修回日期:2021-04-15)
(编辑:陈 宏)
