Evaluation of pig oocyte in vitro maturation and fertilization using three gonadotropin-based hormonal compounds
2021-04-01RosarioSantiagoRodriguezAlmaAlvarezGuerreroFernandoGarciaGonzalezAliciaAlcantarRodriguezAlfredoMedrano
Rosario Santiago-Rodriguez,Alma L.Alvarez-Guerrero,Fernando Garcia-Gonzalez,Alicia Alcantar-Rodriguez,Alfredo Medrano✉
1Autonomous University of Mexico State,Amecameca,Mexico
2Faculty of Sciences,National Autonomous University of Mexico,Mexico
3National Autonomous University of Mexico,FES Cuautitlan:Km 2.5 Carretera Cuautitlan-Teoloyucan,54714 Cuautitlan Izcalli,Estado de Mexico,Mexico
ABSTRACT
KEYWORDS:Chorionic gonadotropins; Embryo; Oocyte maturation; In vitro fertilization
1.Introduction
Oocyte maturation to the metaphase Ⅱ(MⅡ)stage of oogenesis is a complex process that involves restarting the meiotic cycle,as well as the rearrangement of organelles and cytoplasmic components[1].During the growth phase,oocytes acquire cytoplasmic organization that depends on gene expression and the multiplication,modification and redistribution of organelles,in addition to post-transcriptional modification of mRNAs that will be accumulated for later use during embryo segmentation[2].Oocyte growth and the zona pellucida organization depend on the intercellular communication with cumulus cells via gap junctions,which allow the passage of molecules from the follicular environment to the oocyte and vice versa[1].They also depend on glycoprotein release,which allows the oocyte to sustain its own development and accumulate factors for initial embryonic divisions[3,4].These events have been linked to oocyte competition to distinguish oocytes that are capable of producing a viable and transferable embryo after fertilization[5].In assisted reproduction techniques such as in vitro fertilization(IVF),oocytes can be matured either in vivo or in vitro; while sperm must undergo an in vitro sperm capacitation process.Both gametes must be evaluated in order to achieve fertilization[6].
In domestic animals,and specifically in pigs,IVF system development has become increasingly important in light of a wide range of envisioned possible uses[7].These include the production of transgenic animals for xenotransplantation,procuring stem cells,and research on gene therapy and experimental biomedicine[8].However,as IVF performance is far from 100% and well below the success rate attained under natural fertilization conditions,techniques are under continuous evolution and remain the subject of research[9].In an attempt to improve oocyte viability and maturation,the addition of various natural supplements,proteins,energy substrates,antioxidants[10],hormones and growth factors to culture media has been tested in order to achieve acceptable rates of follicular growth,viability and follicular maturation[11].
In vitro maturation is frequently carried out in media supplemented with chorionic gonadotropins or with follicle stimulating hormone(FSH)and luteinizing hormone(LH)to induce cumulus cell expansion and nuclear maturation[12].Different commercial hormone products have been used as alternatives to induce estrus and improve in vivo ovulation rates in domestic animals.Chorulon[5 000 IU human chorionic gonadotropins(hCG),mainly LH]has been used in sows to induce ovulation,stimulate follicle maturation,and to maintain the functional life and increase progesterone secretion by the corpus luteum.Novormon[5 000 IU equine chorionic gonadotropins(eCG)]is another product frequently used in vivo,given that its dual action(FSH/LH),stimulates follicular development and ovulation in most domestic species.Novormon administration stimulates follicular development and enhances the action of progestins,achieving heat synchronization in sows,cows and sheep[13].PG600(400 IU eCG / 200 IU hCG)is used in adult and nulliparous sows to induce and synchronize heat,and has a fundamental role in maintaining the early stages of gestation[13].Because these products represent a viable option for improving in vitro oocyte maturation in domestic animals,our research aimed to compare the effect of the three hormonal compounds(Novormon,PG600,and Chorulon)on the in vitro maturation of sow oocytes obtained from sows slaughtered in an abattoir.
2.Materials and methods
2.1.Collection of ovaries and cumulus-oocyte-complexes
Experimental work was carried out by using ovaries obtained from pre-pubertal gilts slaughtered at abattoir and then transported to the laboratory in a physiological saline solution(0.9% NaCl w/v)at 30 ℃,within a maximum of two hours.In the laboratory,ovaries were washed twice with physiological saline solution at 30 ℃.Follicular fluid was aspirated from antral follicles of 3-6 mm diameter,via aspiration method using a 10 mL hypodermic syringe without a rubber plunger,and an 18 g needle(Air-tite,Germany).
Follicular fluid was deposited in 50 mL conical tubes and allowed to settle for 20 min to obtain the cellular package.After this time had elapsed,the supernatant was removed and the cellular package was washed with 2 mL of TL-HEPES solution(Tyrode's Lactate-4-(2-Hidroxyethyl)piperazine-1-ethanesulfonic acid,N-(2-Hidroxyethyl)piperazine-N'-(2-ethanesulfonic acid)(In vitro,Mexico),then the suspension was left standing for 15 min and the procedure was repeated once more.Subsequently,the cellular package was placed in a petri dish(100 mm ×15 mm,Falcon,USA)and observed under a stereoscopic microscope with the 7×objective(Nikon,Japan)to search for cumulus-oocyte complexes and evaluate them based on morphology.Cumulus-oocyte complexes were selected according to the De Loos et al[14]classification,and only those of quality 1 to 3(intact zona pelucida,homogeneous appearance of oocyte cytoplasm,and at least 2 to 5 layers of cumulus cells surrounding the oocytes)were used in the experiments.
2.2.In vitro oocyte maturation
A total of 712 oocytes distributed across six replicates(shown in Supplementary Figure 1),each with the whole set of treatments,were worked with during the in vitro maturation experiments.Selected oocytes were washed three times in 500 µL drops of TCM-199 maturing medium(Tissue Culture Medium-199),with bicarbonate and Earle's salts(In vitro,Mexico).To test and compare the effects of the three hormonal compounds(Novormon,PG600,and Chorulon)on in vitro maturation,each of the three hormonal treatments and the control treatment were added to about 30 oocytes and transferred to four-well dishes(Nunc,Denmark)with 495 µL of maturing medium in each well-dish.For the control group,the additional 5 µL of the same maturing medium(500 µL total)was added.For the Novormon treatment group,5 µL of eCG solution(5.0 IU)(Novormon5 000 IU eCG,Virbac,Mexico)was added in each well-dish.For the PG600 treatment group,5 µL solution of eCG(5.0 IU)and hCG(2.5 IU)(PG600Intervet 400 IU eCG/200 IU hCG,MSD,Mexico)was added to each well-dish.For the Chorulon treatment group,5 µL of a solution with hCG(5.0 IU)(Chorulon5 000 IU hCG,MSD,Mexico)was added to each well-dish.Similar doses of these gonadotrophins have been used previously[15-17].In addition,0.5 µg/mL of epidermal growth factor(Sigma E4127)and 0.5 µg/mL of gentamicin(Tornel,Mexico)were added to each well,and finally the wells were covered with 200 µL of mineral oil.The dishes were incubated at 38.5 ℃ with 5% CO,95% air and humidity at saturation for 44 h.
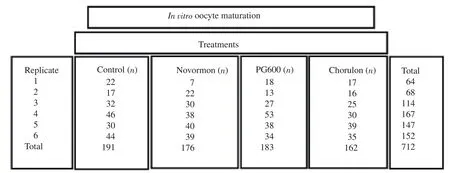
Supplementary Figure 1.The concrete number of oocyte used in each group in in vitro oocyte maturation.
2.3.Evaluation of in vitro maturation
Of the 712 oocytes employed in the first experiment,232 from the four treatments were used to carry out an indirect assessment of oocyte maturation.Likewise,480 oocytes from the four treatments were used to carry out a second assessment of oocyte maturation by means of the aceto-orcein staining.
The inverted microscope with the 40×objective(Leica DMIL LED,Germany)was used to carry out a primary indirect evaluation of in vitro maturation of gilt oocytes to second metaphase(MⅡ); the selected oocytes were with uniform and homogeneous cytoplasm and expanded cumulus cells.This was done prior to a second evaluation by means of the 1% aceto-orcein technique.For this,20 oocytes from each of the four treatments were deposited on a slide and pressed by using a coverslip with drops of a paraffin and petrolatum(1:1)mixture used as a cementing material.Subsequently,pressed oocytes were fixed in a glacial acetic acid-ethanol solution(1:3 v/v)(JT Baker,Mexico)for 72 h and then oocytes were stained with a 1% orcein solution in 45% acetic acid; slides were evaluated by using an optical microscope with the 40×objective(Leica ICC50 HD,Germany).
Oocytes displaying a germinal vesicle were considered as immature,those observed in the first metaphase stage(MⅠ)were considered to be maturing,and those that were in the MⅡ and/or presented a polar body were classified as mature[18].Oocytes in which the chromatin-stained nucleus could not be observed and/or without the presence of a first polar body were considered undefined.
2.4.In vitro fertilization
A total of 741 oocytes,distributed across six replicates for each treatment(shown in Supplementary Figure 2),were worked with during the second experiment.Oocyte maturation and assessment were carried out as mentioned,but matured oocytes were transferred to in vitro culture to continue ongoing stages of fertilization and embryonic development to see whether supplementing maturation medium with chorionic gonadotropins may help those oocytes to become fertile and generate viable embryos up to blastocyst stage.Following in vitro maturation,cumulus cells were mechanically removed by using a glass Pasteur pipette.Naked oocytes were washed twice with fertilization medium(In vitro,Mexico)supplemented with 6% bovine serum albumin with fraction Ⅴ fatty acids(Sigma,USA),and 0.5 µL/mL gentamicin(Tornel,Mexico).For in vitro fertilization,30 to 40 matured oocytes were placed in four-well dishes containing 500 µL of fertilization medium and 200 µL of mineral oil(Fisher Scientific,USA),and incubated at 38.5 ℃ with 5% CO,95% air and humidity at saturation.

Supplementary Figure 2.The concrete number of oocyte used in each group in in vitro fertilization and embryonic development.
Semen was manually obtained from sexually mature pigs and then processed and frozen in the Animal Reproduction Laboratory(L2-UIM).Straws were kept frozen in liquid nitrogen until the day of in vitro fertilization,at which point each straw was thawed in a thermoregulatory bath at 38 ℃ for 3 min.Sperm viability was based on progressive motility,and then 1.0 mL of the semen sample was placed in a 15 mL Falcon tube(Biologix)and centrifuged at 700×g for 20 min.Then the supernatant was discarded and the button containing the sperm package was reconstituted with 1.0 mL of TL-HEPES solution(In vitro,Mexico)previously incubated at 37 ℃,and transferred to a 1.5 mL microcentrifuge tube.Sperm concentration was estimated by adding 10 µL of sperm and HEPES solution(1:200 dilutions)to a test tube containing 1 990 µL of 0.3% formalin saline solution.After allowing the suspension to settle for 5 min,we carried out a count in a Neubauer chamber(Hausser Scientific,Germany).We added 1×10sperm/mL of fertilizer medium,and then carried out in vitro fertilization in four-well dishes.Each well contained 500 µL of fertilization medium and 10 µL of the sperm suspension; sperm and oocytes were co-incubated for 7 h.
2.5.In vitro embryonic development
After the co-incubation period(in vitro fertilization),potential zygotes were washed three times with NCSU-23 development medium(North Carolina State University-23)supplemented with 4% bovine serum albumin with fraction Ⅴ fatty acids.We transferred 20 to 25 probable zygotes to four-well dishes with 500 µL of the same medium and covered them with 200 µL of mineral oil,and incubated at 38.5 ℃ with 5% CO,95% air and humidity at saturation.After developing for 7 days,the embryos were evaluated by using the classification of the International Embryo Transfer Association[19].Embryos were evaluated using the inverted microscope with the 40×objective(Leica DMIL LED,Germany).
2.6.Statistical analysis
Percentages were obtained by considering the total number of oocytes used in each of the treatments(and replicates)of in vitro maturation,and the subsequent number of(i)mature,(ii)maturing,(iii)immature,and(iv)undefined oocytes; each of these numbers were divided by the total number of oocytes from each treatment,and the resulting number was multiplied by 100.
Percentages of the different stages of embryonic development:(i)2 to 12 cells embryos,(ii)morulae,(iii)compact morulae,(iv)young blastocysts,(v)mature blastocysts,(vi)expanded blastocysts,(vii)degenerated,and(viii)undefined embryos were obtained from the number of fertilized oocytes in each of the treatments(and replicates); each of these numbers were divided by the total number of fertilized oocytes from each treatment,and the resulting number was multiplied by 100.
Data from the different experiments(i.e.,oocyte maturation,and embryo development)were analyzed by analysis of variance(ANOVA)to look for differences between treatments; post hoc comparisons were carried out by Tukey´s test.Data were arcsine transformed to normalize them before the ANOVA.Percentages were calculated for each variable in each replicate; then,these values(each variable from each of the six replicates)were used for the ANOVA.Thus,each percentage(±SD)presented in the tables was the mean value of those individual percentages from the six replicates.IBM SPSS Statistics v22(IBM Corp)was employed for these analyses; results were presented as(i)mean±standard deviation(mean±SD),and(ii)median(quartiles 25-75).The level of significance was at P<0.05.
2.7.Ethics statement
This work was not subjected to the Subcommittee for Care of Animals in Experimentation from the National Autonomous University of Mexico since it did not involve direct work with live animals but only ovaries and oocytes collected postmortem.
3.Results
3.1.In vitro maturation
The Novormon treatment group produced the highest percentage of oocytes in MⅡ stage,followed by PG600 treatment group while these values were not significant different from each other(P>0.05).All the three treatment groups showed higher percentages of oocytes in MⅡ stage than the control group(P<0.05),but there was no significant difference among the three groups(Table 1).
The control group had the highest proportion of MⅠ oocytes as compared with the Novormon,PG600,and Chorulon treatment groups(P all <0.05),while there were no statistically differences among the Novormon,PG600,and Chorulon treatment groups(Table 1).
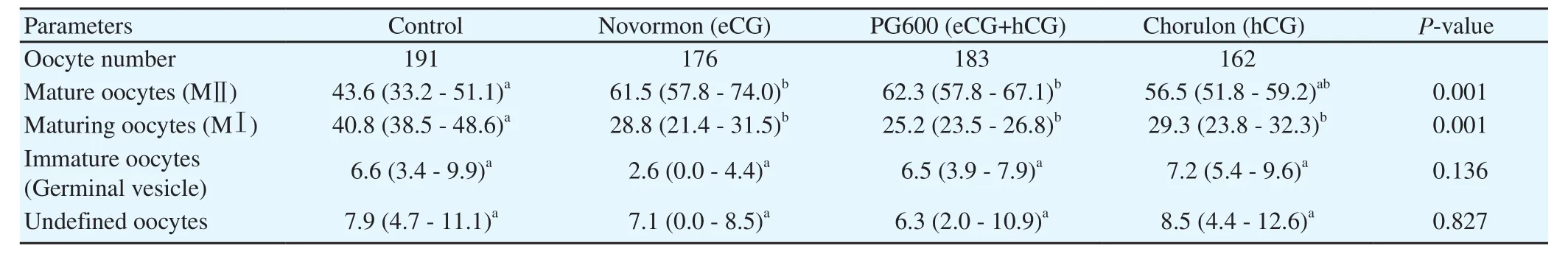
Table 1.Effect of different gonadotropin-based treatments on pig oocyte in vitro maturation.
No significant differences were found in oocytes in the germinal vesicle stage or the undefined oocytes among the control,Novormon,PG600,and Chorulon treatment groups(P all > 0.05).
3.2.In vitro fertilization and embryonic development
While all treatments yielded embryos at different developmental stages,the developmental delays at day 7 of the embryonic development resulted in very few expanded blastocysts and none hatched ones(Table 2).No difference in percent fertilization was observed among the control,Novormon,PG600,and Chorulon treatment groups(Table 2).
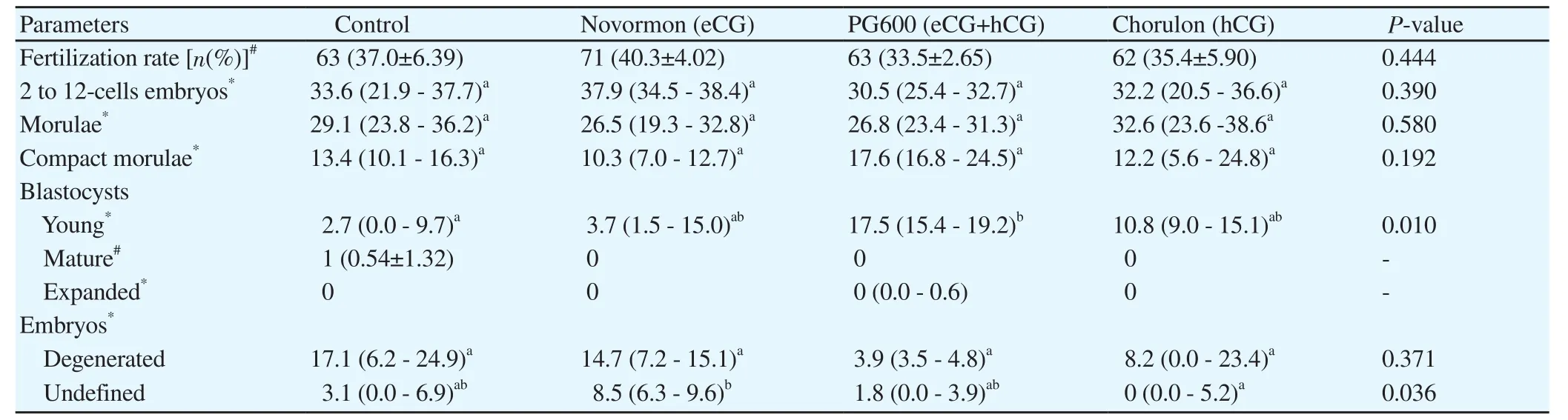
Table 2.In vitro fertilization and embryonic development of pig oocytes matured with different gonadotropin-based treatments.
There were no significant differences in the percentage of 2 to 12-cell embryos among the control,Novormon,PG600,and Chorulon treatment groups(P=0.390).No significant differences were recorded between treatments regarding the percentage of early morulae for the control,Novormon,PG600,and Chorulon treatment groups(P=0.580),nor between treatments for the compact morula stage of the control,Novormon,PG600,and Chorulon treatment groups(P=0.192).
A significant increase in the young blastocyst stage of the PG600 treatment group as compared with the control group(P<0.05).Percentage of young blastocysts in the Novormon and treatment groups were not significant different from that of the control group.The mature blastocyst was found only in the control group,while an expanded blastocyst developed in the PG600 treatment group.The degenerated embryos did not vary significantly among the four groups(P=0.371).The undefined embryos in the Novormon,PG600,and Chorulon treatment groups were not different from the control group.A significant increase in undefined embryos was found between the Novormon and Chorulon treatment groups(P<0.05),but PG600 treatment group did not differ from the other treatment groups(Table 2).
4.Discussion
Under in vitro conditions,there is an absence or scarcity of growth factors produced by the oocyte such as growth factor beta,growth differentiation factor and bone morphogenetic protein 15[20];additionally,the cyclic adenosine monophosphate exchange is altered due to oocyte extraction from the follicle.These factors,together with physicochemical management processes,are decisive in achieving adequate nuclear and cytoplasmic maturation[21].Hormonal treatments for embryonic maturation can produce variable results due to a series of causes including the characteristics of the hormone used,heterogeneity of oocytes sourced from ovaries from abattoirs,the response of each individual[22]and the effect of reactive oxygen species[23].
In vivo experiments in sows have shown that eGC and hGC application induces maturation and synchronization of post-weaning estrus.This is associated with increased ovulation numbers in primiparous sows subjected to short lactations[24].Therefore,in vitro processes are considered to be stressful for the oocyte.Despite this stress,this study found treatments with Novormon and PG600 obtained a significant increase in oocyte maturation(64.6% and 62.3% respectively)as compared with the control group(41.9%).This denotes the efficiency of the hormonal products mentioned in in vitro maturation and coincides with Meinecke and Meinecke-Tillman[25],who showed that adding 2 IU of eCG or 2 mg/mL of FSH to the maturing medium of in vitro sow oocytes,allowed them to obtain 87% of MⅡ oocytes.However,cumulus mass expansion was only observed in media containing eCG and not in media supplemented with hCG.In our study,the best results with eCG(Novormon)(64.6%)were also observed at this stage.Additionally,it has been published that the optimal dose of hCG in women should be individualized depending on each situation and patient,since no homogeneous results have been found with the use of this hormone[26].This is probably reflected in our results,as the Chorulon(hCG)(55.6%)and control(41.9%)treatments had the lowest percentages of oocyte maturation.It would be interesting to perform individual dosages in domestic animals.
Regarding the proportion of oocytes in the maturation process(MⅠ),we found that the three gonadotropin treatments(Novormon,PG600 and Chorulon)shared similar behavior with each other(27.4%,25.2% and 28.7% respectively)and were statistically different when compared to the hormone-free treatment(the control group)(44.2%).These results disagree with those reported by Romar et al[27],who observed that the percentage of maturing sow oocytes(43.0%)was not affected by absence of hormone use.They attributed this effect to the presence and permanence of cortical granules in oocyte cytoplasm,which allow for favorable oocyte maturation.
This observation suggests that the proportion of maturation in MI in the treatment without hormones(44.2%)indicate that in addition to hormonal activity,factors intrinsic to the oocyte may be promoting maturation.It has been reported that GnRH(Gonadotropin-Releasing Hormone)in women can generate a gonadotropin surge and lead to 85% follicular maturation in vitro,which is not observed with the use of hCG(58%)[28].Similarly,in our study,hCG(Chorulon)showed low maturation percentages(55.6%)when compared to eCG(Novormon,64.6%).
Some commercial products used in veterinary medicine are partially purified eCG preparations,made from the blood of pregnant mares.This carries with it the disadvantage of great variability between batches[13]; this could be another factor that affects the efficiency of this in vitro product.
Hormonal treatments based on eGC and hCG have been tested in rats,and it has been observed that the ovulation rate was significantly higher with the use of eGC(22.3%)as compared with the use of hCG(14%)[23].This coincides with our results as we observed the best effects during maturation when using eCG alone(64.6%)or combined with hCG(62.3%).
In our research,in vitro fertilization was performed with frozen semen,and the highest(but no significant)fertilization percentage was with Novormon treatment(eCG,40.3%),followed by the hormone-free treatment(37.0%).Our results coincide with those obtained by Budiyanto et al[29]who used frozen semen and matured sow oocytes(44.5%),adding follicular fluid as a supplement.Likewise,Romar et al[27]reported a 30% fertilization rate by adding follicular fluid and without the use of hormones.
Regarding embryonic development in 2 to 12-cell embryos,the highest percentage was obtained with the Novormon treatment(eCG,36.7%)followed by the control treatment(30.9%); the percentages were observed with the PG600(29.6%)and Chorulon(29.2%)treatments were lower.These results are similar to those reported by Yoshida et al[30]who obtained 24% of sow embryos at this stage without the use of hormones.
In vitro maturation,fertilization and embryo culture techniques maintain low development rates up to the blastocyst stage,and the embryos produced may have low implantation rates as a consequence of failures during the oocyte maturation process[31].We found significant differences in the young blastocyst stage between the control group(5.3%)and the PG600 treatment group(17.5%).These results are similar to those reported by Budiyanto et al[29],in whose work the maturing medium was supplemented with follicular fluid,and they obtained blastocysts(4.4%)without the use of hormones.Funahashi et al[32]observed two blastocysts matured on day 6 using 199 medium,which is very similar to our results:one blastocyst matured with the control treatment and one expanded blastocyst with the PG600 treatment using NCSU-23 medium.
In general,blastocysts produced in vitro present high rates of DNA fragmentation,apoptotic cells,impaired embryo metabolism[33,34]and chromosomal abnormalities[35].We found the lowest percentage of degenerated cells was obtained with the PG600 treatment(4.2%),while the control treatment produced the highest percentage of fragmentation(16.2%).This suggests a positive hormonal effect.
The percentage of undefined embryos differed between the Chorulon(1.95%)and Novormon(8.0%)treatment groups.Given the opaque nature of porcine oocyte cytoplasm,structures such as cortical granules cannot be observed with conventional microscopy.The first descriptive studies of swine oocytes were carried out with electron microscopy[36].Later,Yoshida et al[30]used confocal laser microscopy and five different lectins specifically to stain the cortical granules.This non-invasive analysis allowed the recording of virtual optical planes,revealing spatial and spectral information not available by using classical light microscopy.
In conclusion,hormonal products Novormon(eCG)and PG600(eCG+hCG)allowed us to obtain the highest percentages of in vitro maturation in gilt oocytes; however,this effect was not transferred to fertilization rates.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Funding
This research was completed through several grants from Universidad Nacional Autonoma de Mexico(PAPIIT IN220419,IN219620,and PIAPI 1810,2030).
Authors' contributions
Alfredo Medrano,Fernando Garcia-Gonzalez,and Alma L.Alvarez-Guerrero designed the study,supervised the experimental work,analysed the data,and drafted the paper.Rosario Santiago-Rodriguez,Alma L.Alvarez-Guerrero,Fernando Garcia-Gonzalez,and Alicia Alcantar-Rodriguez carried out the experimental work,collected,organized,and analysed the data,and drafted the manuscript.
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- Association between estradiol levels and clinical outcomes of IVF cycles with single blastocyst embryo transfer
- Antifertility effects of 60-day oral gavage of ethanol extract of Spondias mombin leaves in guinea pigs:A biochemical,reproductive and histological study
- Combined effects of Gymnema sylvestre and Pergularia daemia on letrozole-induced polycystic ovarian syndrome in rats
- Aphrodisiac potential of Polyalthia bullata(Tongkat Ali)in fowl
- Semen characteristics of the three genetic types of boars reared in Benin
