STAT3 Inhibition by Centipede Scolopendra Extract in Liver Cancer HepG2 Cells and Orthotopic Mouse Models of Hepatocellular Carcinoma
2020-09-26TENGYongJieLIUZhuoLIAOLiuCHENYunHUANGXioDiTIANXueFei
TENG Yong-Jie,LIU Zhuo,LIAO Liu,CHEN Yun,HUANG Xio-Di,TIAN Xue-Fei*
a.The First Hospital of Hunan University of Chinese Medicine,Changsha,Hunan 410007,China
b.The Affiliated Hospital of Hunan Academy of Chinese Medicine,Changsha,Hunan 410006,China
c.College of Integrated Traditional Chinese and Western Medicine,Hunan University of Chinese Medicine,Changsha,Hunan 410208,China
d.Hunan Key Laboratory of TCM Prescription and Syndromes Translational Medicine,Hunan University of Chinese Medicine,Changsha,Hunan 410208,China
Keywords
Centipede Scolopendra extract (CSE)
Liver cancer
Nude mice
Protein tyrosine kinase (PTK)
STAT3
Protein chip
ABSTRACT
Objective To observe the effects of Centipede Scolopendra extraction (CSE) on human liver cancer HepG2 cells and the nude mouse tumor model of liver orthotopic transplantation,and to explore the anti-liver cancer mechanism of the extract.
Methods HepG2 cells were respectively treated with CSE250 (250 μg/mL),CSE500 (500 μg/mL) and 5-FU,and control group was established.An enzymatic hydrolysis and acetone precipitation method was used to separate and purify CSE,which was then used to treat HepG2 cells.The CCK8 assay was used to detect the inhibition of cell proliferation and the half maximal inhibitory concentration (IC50) was calculated.Flow cytometry was used to analyze the cell cycle,and western blot was used to detect the expression of signal transduction and activator of transcription 3 (STAT3) pathway-related proteins in HepG2 cells treated with CSE.A nude mouse model with an orthotopic liver tumor was prepared.The mice were randomly divided into four groups,each containing 12 animals:the model group,the 5-FU group,the CSE10 group [10 mg/(kg·d)] and the CSE50 group[50 mg/(kg·d)].The volume and mass changes in the nude mice with orthotopic transplanted tumors were observed.Western blot method was used to test the protein expression levels of p-STAT3 and p38 mitogen-activated protein kinase (p38MAPK).Tissues from the liver of mice in the model group and the CSE50 group were analyzed by using a protein tyrosine kinase (PTK) chip,and the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) function enrichment analysis of the differentially expressed proteins was performed.
Results This study showed that CSE significantly inhibited the proliferation of HepG2 cells (P < 0.05).After 48 h of CSE treatment,the cell cycle of HepG2 cells manifested as S phase and G2/M phase; p-STAT3 protein levels in the CSE groups were significantly lower than that in the control group (P < 0.05).Analysis of the tumor inhibition in the mice showed that the tumor masses and volume in CSE groups were lower (P < 0.05).The protein levels of p-STAT3 and p38MAPK in CSE50 group and 5-FU group decreased significantly (P < 0.05).PTK antibody chip screening results showed that CSE groups had a bidirectional regulation trend,and there were 23 up-regulated PTKs and six down-regulated PTKs.The GO and KEGG analyses showed that CSE exerted its anticancer effects through regulation of biological processes,including mitogen-activated protein kinase (MAPK)cascade,chemotaxis,cell invasion,cell adhesion,angiogenesis and other biological processes,and through signaling pathways,including the MAPK,phosphatidylinositol-3-kinase/serine threonine protein kinase (PI3K/AKT),and RAS signaling pathways.
Conclusions CSE can effectively inhibite the proliferation of HepG2 cells and effectively inhibite the growth of liver cancer orthotopic transplantation tumor.Its mechanism may be closely related to the regulation of STAT3,MAPK and PI3K/AKT signaling pathways.
1 Introduction
Hepatocellular carcinoma (HCC) is currently the third most common cause of cancer death in China[1].Its high malignancy and heterogeneity make the treatment of HCC extremely challenging.Although sorafenib was approved for the treatment of advanced liver cancer in 2007[2],clinical trials suggested that it did not improve patients’ overall survival[3].More recently,other small molecule multikinase inhibitors,such as regorafenib,lenvatinib and cabozantinib,have been approved for the treatment of liver cancer.However,these treatment options are far from satisfactory owing to toxicity and the rapid progression of drug resistance.Therefore,it is important to identify effective medications against HCC.
The use of traditional Chinese medicine (TCM) in liver cancer treatment has been reported widely[4,5].TCM believes that “deficiency,stasis and toxicity” are the basic pathogeneses of liver cancer; therefore,treatment focuses on strengthening the resistance of liver and spleen,in addition to removing blood stasis,detoxicating and dissolving lumps.TCM is effective in alleviating clinical symptoms,improving qualityof-life and extending survival.Prescription analysis indicated that prescriptions aimed at detoxicating and dissolving tumor mass often consist of insects,such as centipede,scorpion,ground beetle and leech.Modern research has found that insect medicine as shown a superior antitumor effect,the mechanism of which is associated with the inhibition of tumor cell proliferation,the promotion of apoptosis,and restoring the tumor microenvironment to a normal state[6].However,these drugs often have a strong efficacy or a slight toxicity,and inappropriate use of these may lead to impairment of vital Qi and stomach Qi.In addition,the uncharacterized clinical nature of insect medicine make most clinicians reluctant to use them.To address this issue,we performed a series of studies on the antitumor mechanisms of insect medicines,from which we found that centipede extract exhibited the most significant antiliver cancer efficacy[7].In this study,we have performed furtherin vitroandin vivoinvestigations into the effects of Centipede Scolopendra extract (CSE) on hepatoma cells and in nude mice orthotopically transplanted with a liver tumor,respectively.In addition,we used protein chip technology to examine the molecular mechanism of CSE from a bioinformatics perspective.
2 Materials and Methods
2.1 Animals and cell lines
This study used SPF-grade pure BALB/C-nu/nu male nude mice.The mice were 4-6 weeks old and had a weight of (20±3) g.The mice were provided by Hunan SJA Laboratory Animal Co.,Ltd.(Hunan,China),license number:SCXK-Hunan-2009-0004.The mice were subsequently raised in the SPF-grade laboratory of the Animal Experiment Center of Hunan University of Chinese Medicine.
Human liver cancer HepG2 cell lines were purchased from the Cell Center of Xiangya School of Medicine.These cells were placed in a high glucose DMEM (GIBCO,Waltham,MA,USA) containing 12%fetal bovine serum (FBS) (Invitrogen,Carlsbad,CA,USA) and cultured in a constant temperature incubator at 37 ℃ and 5% CO2with saturating humidity.
2.2 Drug preparation
CSE was prepared by the enzymatic hydrolysis and acetone precipitation method[8].The centipede crude drug,purchased from the Department of Pharmacy,the First Hospital of Human University of Chinese Medicine,was first identified.The crude drug was then prepared into ultrafine powder with a particle size of 1-75 μm by using the enzymatic hydrolysis and acetone precipitation method,and frozen at − 20 ℃.Subsequently,2 g of ultrafine centipede powder was accurately weighed and protease-hydrolyzed at 55 ℃ before it was inactivated in a 99 ℃ water bath for 10 min.The solution was then centrifuged at 4 ℃ and 5 000 r/min or 10 min;subsequently,the supernatant was extracted,cooled and precipitated in two volumes of acetone.Next,the solution was further centrifuged at 4 ℃ and 5 000 r/min for 10 min,and the precipitate was then completely dissolved in ultrapure water.This step was repeated twice; the final precipitate was collected,dissolved in ultrapure water,precooled,and precipitated in two volumes of acetone.The precipitate of the last step was then extracted,vacuum-dried into a powder at 30 ℃,and then frozen at − 20 ℃ for subsequent use.
2.3 In vitro test indicator
2.3.1 Grouping and cell proliferation inhibition rate measurementHepG2 cells in the logarithmic growth phase were prepared as a non-aggregated cell suspension at a concentration of 5×104cells/mL and 200 μL per well was inoculated in a 96-well plate.Five concentrations of CSE intervention (1.5,0.75,0.375,0.188 and 0.094 mg/mL),as well as a control group,and a background group (a blank well),were analyzed; five duplicate wells were used for each measurement.Complete medium was added to wells in the control group after cell inoculation,whereas only the complete medium (i.e.,without cells) was added to the wells in the background group.They were cultivated in the medium for 24 h,48 h and 72 h respectively.Subsequently,the CCK8 assay (7sea Biotechnology Co.,Ltd.,Shanghai,China) was performed in accordance with the manufacturer’s instructions,and the percentage cell proliferation inhibition rate was calculated using the formula:[1 –(OD value of the CSE group/OD value of the control group)]×100%.The half maximal inhibitory concentration (IC50) was calculated by using the linear regression equation.
2.3.2 Cell cycle detectionHepG2 cells in the logarithmic growth phase were prepared as a nonaggregated cell suspension at a concentration of 1×105cells/mL and inoculated into a 6-well plate.The CSE group and a control group were examined.Subsequently,cells in the CSE group were treated with the CSE solution at the IC50,whereas cells in the control group were treated with an equal volume(2 mL) of complete medium.After 48 h of CSE treatment,the cell cycles of both groups were detected by using flow cytometry and propidium iodide (PI) staining.
2.3.3 Detection of STAT3 pathway protein expressionWestern blot was used to detect the expression of proteins related to the STAT3 pathway in HepG2 cells treated with CSE.First,HepG2 cells in the logarithmic growth phase were prepared as a non-aggregated suspension at a concentration of 2×106cells/mL and 500 μL was inoculated in a 6-well plate.When the cells had adhered to the plate,250 or 500 μg/mL CSE was added,and a control group and a 5-FU group (10 μg/mL) were also established.After 48 h of treatment,the cells were collected and washed with PBS buffer at 0 ℃.Subsequently,lysis buffer(containing 2.5% phosphatase inhibitor mixture and 1% NP-40) was added,and the cells were placed on ice for 30 min until lysis was complete.Finally,after sonication at 500 W,the cells were centrifuged at 4 ℃and 14 000 r/min for 5 min,and the supernatant was used for assay of the total protein of the sample.The protein concentration in the supernatant was determined by using BCA assay.In addition,western blot was used to detect the protein expression of STAT3 (Cell Signaling Technology,rabbit),p-STAT3(Cell Signaling Technology,mouse),matrix metalloproteinase-2 (MMP-2)(Cell Signaling Technology,rabbit),and vascular endothelial growth factor (VEGF)(Abcam,rabbit).A primary antibody dilution of 1:1 000 was used and a secondary antibody(ProteinTech,HRP-conjugated goat anti-rabbit,goat anti-mouse) diluted of 1:5 000 was used.After the membrane was washed,the hybridization signal was detected by using the Amersham ECL luminescence kit (GE Healthcare,Chicago,IL,USA).Images of the gel were collected by using the Rio-Rad gel imaging system,and the images were processed in the Quantity One software to analyze the gray value.The entire experiment was repeated three times.
2.4 Animal model preparation and group drug administration
2.4.1 Preparation of nude mice with orthotopic liver tumorsIn this study,the nude mice received an orthotopic liver transplant by the same method proposed by TAO et al.[9]HepG2 cells in the logarithmic growth phase were prepared as a solutions at a cell concentration of 2×106cell/mL.Subsequently,the cell solution was inoculated subcutaneously in the back of 10 nude mice at a volume of 0.2 mL per site,and the conditions of the nude mice and the transplanted tumors were monitored.When the subcutaneously transplanted tumor in the nude mice reached a diameter of 1.0 cm,the mouse was sacrificed,and the tumor was removed.The tumor was dissected and cut into 1 mm3tissue blocks for subsequent use.Next,a nude mouse liver tumor orthotopic transplantation model was established.First,the nude mouse was anesthetized intraperitoneally with 10% chloral hydrate.An oblique incision was cut at the right costal margin to expose the left outer lobe of the liver,and a 1-2 mm deep tunnel was created to transplant a subcutaneous tumor tissue block into the parenchyma of the left lobe of liver using a puncture needle.Upon completion of the transplantation,a gelatin sponge was used to stop bleeding,and the abdomen was closed layer by layer.After the operation,the mice were allowed free access to food and water,and their growth was monitored daily.
2.4.2 Animal grouping and interventionIn total,55 nude mice received the orthotopic liver transplant.After 7 d,50 mice survived.Of these mice,two were randomly selected,sacrificed,and used to confirm the model was successful established by HE staining.Once confirmed,the remaining 48 nude mice were randomly divided into four groups:the model group,the 5-FU group,the CSE10group and the CSE50group;each contained 12 nude mice.A control group was also established by selecting 12 normal nude mice.The total number of groups was five.On the 15thday after the model was established,drug administration was initiated via intraperitoneal injection,the dosage of which was determined according to the mousehuman mass dose conversion equation.The dosage in the CSE10group was 10 mg/(kg·d) (the crude drug concentration was 5 mg/mL),and the dosage in the CSE50group was five times higher,i.e.,50 mg/(kg·d)(the crude drug concentration remained 5 mg/mL).In both groups,the drug was administered for a period of 14 d.The dosage in the 5-FU group was 50 mg/kg·time(the crude drug concentration was 5 mg/mL),twice per week for a total duration of 2 weeks.Mice in the control and the model group were administered a 0.9% NaCl solution at 10 g/(kg·d) for 2 weeks via intraperitoneal injection.All nude mice were sacrificed after 2 weeks,and the tumor samples were extracted for the detection of their volumes and masses.The levels of p-STAT3 and p38 mitogenactivated protein kinase (p38MAPK) protein in each group were detected by western blot method.
2.5 In vivo test indicators
2.5.1 Tumor suppression rateAfter the nude mice were sacrificed by cutting off the head,the liver orthotopic transplantation tumor was extracted,and the fascia was removed.Subsequently,once the tumor mass was recorded,the long diameter (a) and the short diameter (b) of the tumor were measured by a Vernier caliper,and the tumor volume and the tumor suppression rate were calculated using the following equations:Tumor volume=ab2/2; Tumor suppression rate (%)=[(average tumor mass in the control group – average tumor mass in the admini stration group)/average tumor mass in the control group]×100%.
2.5.2 Protein tyrosine kinase (PTK) chip assayTissues from the liver of the mice in the model group and the CSE50group were collected and preserved in liquid nitrogen for the PTK antibody chip assay.For analysis,the total protein content of the liver tissues frozen in liquid nitrogen was extracted.First,the collected liver cancer tissue was crushed.An extraction reagent containing protease inhibitor was added to extract the proteins,and the extracted proteins were then stored at − 80 ℃ for subsequent use.The protein concentration of the sample was detected by using the Bradford assay at a wavelength of 562 nm and diluted bovine serum albumin (BSA)standards.For the PTK chip assay,the RayBio human protein tyrosine kinase chip membrane (RayBiotech,Norcross,GA,USA) was selected,which could simultaneously detect multiple PTKs including receptor tyrosine kinase (RTKs) and non-receptor tyrosine kinase (nrPTKs).One milliliter of sample was added,the protein chip membrane was sealed,and the chip was incubated at 22-25℃.The membrane was washed,and the primary biotin-labelled antibody was added an incubated at room temperature.The membrane was washed again,and 1:1 000 diluted horseradish peroxidase (HRP)-labeled antistreptavidin antibody and incubated at room temperature.The membrane was washed again.Finally,after the protein chip membrane was incubated in the membrane reaction solution for 2 h at room temperature,it was exposed to X-rays,and the image was scanned and analyzed by using ScanAlyze software to acquire the standardized gray value.The gray value in each group was then compared to identify the upregulated and downregulated proteins.
2.5.3 Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysisMetascape was utilized to perform an enrichment analysis of both the pathway and the process of differentially expressed PTKs.Functional annotation was conducted based on the online tools of Metascape,including the analysis of biological processes,cellular components and molecular functions from GO,as well as the analysis of biological functions and signaling pathways from the KEGG.An entry was significant only when it hadP< 0.01,a minimum count ≥ 3,and an enrichment factor > 1.5.The most statistically significant terms were subsequently selected as representatives and presented in a network diagram to determine the correlations between different terms.Finally,an enrichment analysis of the signaling pathways was performed by using the KEGG Mapper to plot a histogram of the enrichment pathways.
2.6 Statistical analysis
In this study,the SPSS 23.0 software was used for all data analysis.Measurement data were expressed as“±s”.Comparisons between multiple groups were performed using one-way analysis of variance.Pairwise comparisons with homogeneous variance were conducted using the LSD method,whereas those with heterogeneous variance were performed by using Dunnet’s T3 method.P< 0.05 was considered statistically significant.
3 Results
3.1 Inhibitory effects of CSE on human liver cancer HepG2 cell proliferation
The results of the CCK8 method showed that the results of CCK8 method showed that CSE significantly inhibited the proliferation of HepG2 cells (P< 0.05) in a dose-dependent manner (see Figure 1).Compared with 24 h and 72 h,the proliferation inhibitory effects of centipede extraction increased rapidly with the increase of mass concentration when treated for 48 h.At a drug concentration of 1.5 mg/mL,(70.08%±1.1%)inhibition occurred.Therefore,48 h was selected as the processing time for subsequent experiments.By the calculation,the IC50value was 508.3 μg/mL.Following drug intervention at the IC50,the cell counts in the CSE-treated group at 24 h,48 h and 72 h were (1.67±0.17)×105,(0.70±0.21)×105and(0.15±0.48)×105,respectively.The differences between the three groups were statistically significant(P< 0.05).Alternatively,the cell counts in the control group at 24 h,48 h and 72 h were (1.65±1.41)×105,(3.59±1.19)×105and (5.51±1.52)×105,respectively,which were also significant differences (P< 0.05).When comparing the cell counts in the CSE group to those of the control group,although the difference at 24 h was not statistically significant,those at 48 h and 72 h were both significant (P< 0.05),indicating that CSE exerted an obvious inhibitory effect on HepG2 cell proliferation.In the subsequent cell experiments,the mass concentration of HepG2 cells treated with CSE was determined to be 250 μg/mL (equivalent to 1/2 IC50value at 48 h) and 500 μg/mL (equivalent to IC50value at 48 h),and cell protein expression levels were detected.
3.2 Effects of CSE on HepG2 cell cycle
After 48 h of CSE IC50treatment on human liver cancer HepG2 cells,the cell cycle distribution was significantly changed by flow cytometry detection(Figure 2A).The statistical results are shown in Figure 2B:CSE group (41.6%±0.9%),which was significantly different to that in the control group(73.8%±1.0%) (P< 0.05).In contrast with the control group (19.1%±1.3%,7.1%±1.1%),the proportions of cells in the S phase (35.3%±0.9%) and the G2/M phase (23.7%±0.7%) were both elevated; the differences were statistically significant (P< 0.05)(Figure 2B).
3.3 STAT3 pathway protein expression
Western blot analysis was used to detect the expression of proteins related to the STAT3 pathway.The results showed that after HepG2 cells were treated with CSE for 48 h,although the protein level of STAT3 pathway followed a declining trend,there was no significant difference to the control group(P> 0.05).However,the protein level of p-STAT3 in the CSE250group and CSE500group,as well as the 5-FU group,was significantly lower than that in the control group (P< 0.05); the decrease was largest in the CSE500group.In addition,the difference of protein level of MMP-2 and VEGF in the CSE500group and the 5-FU group was statistically significant (P< 0.05),and a greater reduction in the expression of MMP-2 occurred than in the CSE250group (P< 0.05) (Figure 3).
3.4 Effects of CSE on the mass and volume of liver cancer orthotopic transplantation tumor,as well as the protein level of STAT3 signaling pathway
The tumor mass and volume in the model group were(1.20±0.11) g and (612.63±126.87) mm3,respectively.In contrast,the tumor mass and volume in the 5-FU,CSE10and CSE50groups were significantly smaller than those in the model group (P< 0.05).Of the three groups,the tumor mass (0.45±0.07) g and volume(167.57±30.99) mm3in the CSE50group were the smallest,and were significantly different to those in the CSE10group [(0.75±0.08) g and (357.13±25.61) mm3]and the 5-FU group [(0.83±0.07) g and (504.95±50.56) mm3] (P< 0.05).These findings indicated that CSE was able to inhibit the growth of liver tumor tissue and that the inhibitory effect was positively correlated with dose.In addition,the tumor mass and volume in the CSE10group were also significantly smaller than those in the 5-FU group (P< 0.05).The tumor suppression rates in the CSE50group,the CSE10group and the 5-FU group were 62.5%,37.5%and 30.8%,respectively (See Figure 4A,4B and 4C).Western blot results showed that:compared with the model group,the p-STAT3 and p38MAPK protein levels in the CSE10group decreased,but the difference was not statistically significant (P> 0.05);the p-STAT3 and p38MAPK protein levels in the CSE50group and 5-FU group significantly decreased,the difference was statistically significant (P< 0.05)(See Figure 4D and 4E).
3.5 PTK antibody chip screening results
Compared with those of the model group,the expression of five RTKs and one nrPTK were downregulated in the CSE group.The five downregulated RTKs were:leukocyte tyrosine kinase(LTK),AXL receptor tyrosine kinase (Axl),insulin receptor (INSR),L-ros proto-oncogene tyrosine protein kinase (ROS),and tyrosine kinase containing immunoglobulin and epidermal growth factor homology domain (Tie); the downregulated nrPTK was a member of the Janus kinase (JAK) family.In contrast,the expression of 15 RTKs and 8 nrPTKs was upregulated.The upregulated RTKs included three members of the Erythropoietin produces hepatocyte receptor (Eph) family,two members of the endothelial growth factor receptor (EGFR) family,two members of the platelet-derived growth factor receptor (PDGFR) family,two members of the receptor tyrosine kinase-like orphan receptor (ROR)family,two members of the vascular endothelial growth factor receptor (VEGFR) family,and one member from each of the remaining four RTKs families.The upregulated nrPTKs consisted of two members from the Src family and one member from each of the remaining six nrPTKs families (Table 1).
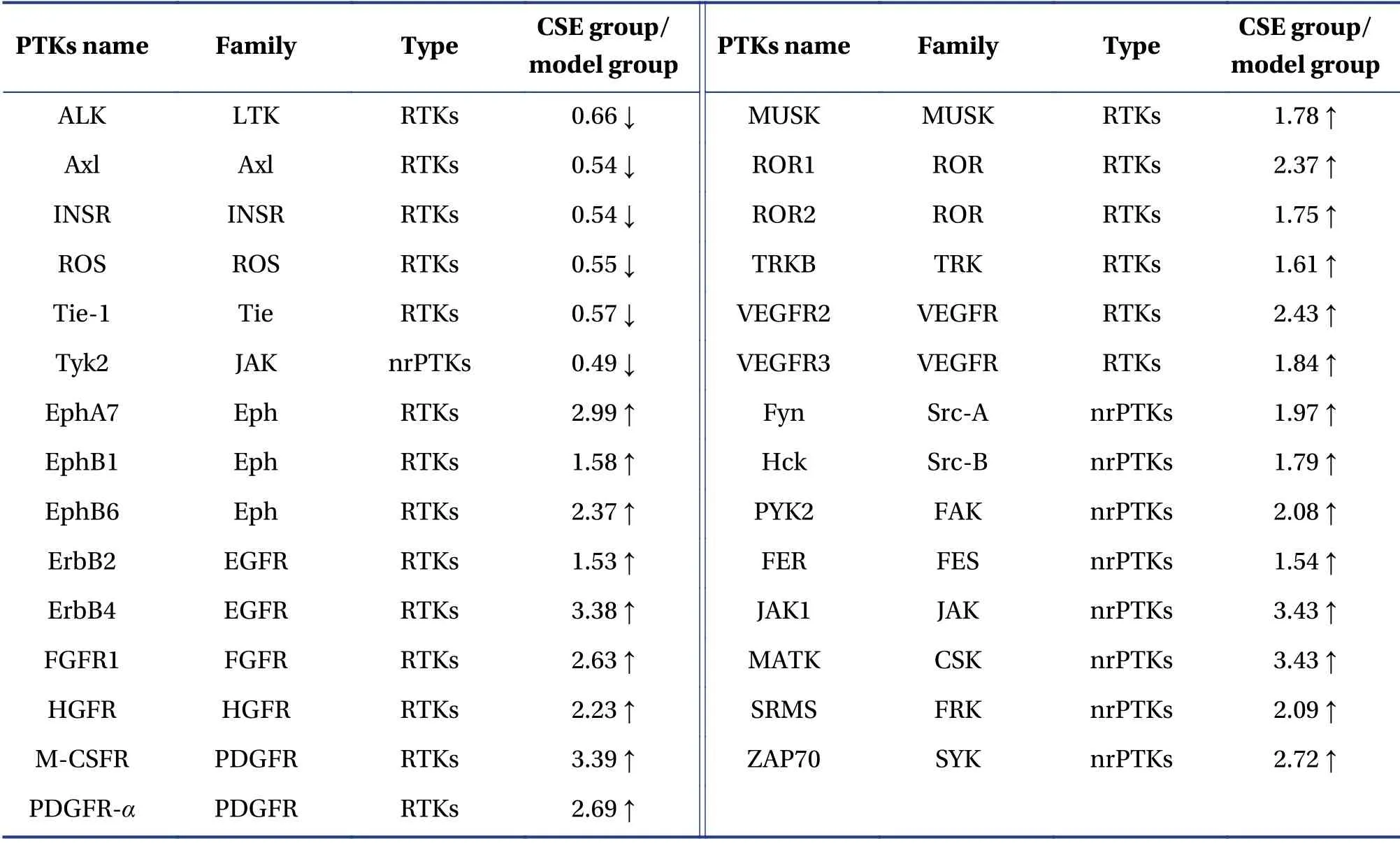
Table 1 Changes in differentially expressed proteins in nude mice orthotopically transplanted with liver tumor and treated with CSE
3.6 Prediction of differential protein’s funtion by GO and KEGG analysis using Metascape
The results suggested that the action mechanism of CSE was primarily correlated with the biological processes of MAPK cascade,chemotaxis,cell invasion,cell adhesion and vascular morphology(Figure 5A).The first 20 KEGG pathways of the differentially expressed proteins are shown in Figure 5B.Of these pathways,the differentially expressed proteins were mainly involved in the PI3K/AKT signaling pathway (as shown in Figure 5D,the differentially expressed protein JAK can directly regulate PI3K),the MAPK signaling pathway,the Ras signaling pathway and the tumor pathway.In addition,these proteins participated in the regulation of the natural killer cell-mediated cytotoxicity,programmed death ligand 1 (PD-L1) expression,and the PD-L1 checkpoint pathway,herpes virus infection associated with Kaposi’s sarcoma,hepatitis B,cell differentiation of Th1,Th2 and Th17,and resistance to EGFR tyrosine kinase inhibitors.As most of these pathways are important mechanisms for tumor initiation and progression,the anti-liver cancer effects of CSE may be mediated through multiple targets.
4 Discussion
As an insect medicine with the capability to remove blood stasis,perform detoxification and dissolve lumps,centipede extracts have been used in the treatment of various tumors,with the achievement of good results[10,11].The TCM expert Professor ZHU Liang-Chun applied centipede extract for the clinical treatment of esophageal cancer,nasopharyngeal cancer,liver cancer,bowel cancer and cancer pain,and obtained good treatment outcomes; on this basis,he suggested that there were many efficacy advantages in the use of centipede extract[10].In addition,the TCM expert ZHOU Zhong-Ying believed that “cancer toxicity” often led to persistent and risky diseases that could only be cured with strong medications.Therefore,he utilized insect medicine,such as centipede and scorpion extracts,to treat cancer based on the concept of “fighting poison with poison”[11].However,the clinical application of centipede extract in cancer treatment is based solely on the individual clinician’s experience:standard application principles and protocols have not been established; usage and dosage are not standardized;and the supporting multi-center clinical research data have not been obtained.The molecular mechanism of the antitumor effect of centipede still requires further clarification.
In this study,we found that CSE inhibited the proliferation of liver cancer cellsin vitro,with clear,dose-dependent inhibitory effects observed after treatment for 48 h.Other domestic scholars have also found similar results of time-dose dependence in experiments on the inhibition of HepG2 cell proliferation by centipede polysaccharides[12].However,the highest inhibition rate in those studies was 56.8%,which was lower than the 70.08% observed in this study.This difference in results may be related to differences in centipede varieties and the extraction methods of the active components.We detected changes in the cell cycle by using PI staining and flow cytometry,and found that treatment with CSE mainly blocked the HepG2 cell cycle in the S phase,suggesting that interfering with DNA synthesis and preventing cell division and proliferation may be one of the anticancer mechanisms.Similarly,studies by SHAN Xiao-Song et al.[13]showed that centipede water extract inhibited the proliferation of tumor cells and prevented cell cycle progression by reducing the expression of proliferating cell nuclear antigen in C6 glioma cellsin vitro.In ourin vivoexperiments,the tumor suppression rate in the CSE10group was 37.5%,whereas that in the CSE50group was 62.5%.WANG et al.[14]found that the tumor suppression rates of S180 solid tumor in mice treated with 0.2,0.4 and 0.6 g/kg doses of Scolopendra were 60.95%,63.68% and 69.01%,respectively; hence,the tumor inhibition effects were similar to those in the CSE50group,but the highest dose was more than 10 times greater than that used in this study,and the tumor suppression rate did not increase significantly when the dose was doubled; this may have be related to the toxicity caused by high-dose Scolopendra .The anticancer effectiveness of CSEin vivohas been confirmed by many studies,but the antitumor effects are dependent on the extraction method,dosage and other factors.The optimal dosage remains unknown and requires further discussion.
Sorafenib,the first systemic drug for HCC,affects a variety of tumor signaling pathways by inhibiting multiple RTKs,including VEGFR1-3,PDGFR,KIT and RET.Like sorafenib,second-line (regorafenib,cabozantinib,nivolumab,pembrolizumab) and new first-line (lenvatinib) targeted therapeutic drugs also inhibit a variety of RTKs and alter the pathogenesis of HCC[3].In our study,the results of antibody chip screening showed that the CSE-treated groups showed a bidirectional regulation trend:there were 23 upregulated PTKs,which included JAK1,EphA7,ErbB4,MATK and M-CSFR; and there were six downregulated PTKs,namely Axl,ALK,Tie-1,INSR,ROS and TYK2.The GO and KEGG analyses showed that CSE exerted its antitumor effects through the regulation of multiple biological processes (e.g.,MAPK cascade,chemotaxis,cell invasion,cell adhesion and angiogenesis) and multiple signaling pathways (e.g.,MAPK signaling pathway,PI3K/AKT signaling pathway and the Ras signaling pathway).The most important regulatory pathways may be the MAPK and PI3K/AKT signaling pathways.
Tyrosine kinase is an important component of the classical MAPK pathway.The main members of MAPK are extracellular regulated protein kinase(ERK),p38MAPK and c-Jun amino terminal kinase(JNK)[15].The activation of the MAPK/ERK pathway counteracts the effects of apoptosis signals arising from upregulated expression of anti-apoptotic proteins,such as Bcl-2,and the inhibition of ERK activity leads to an increase in BimEL expression.Abnormal activation of the MAPK signaling pathway is closely related to the occurrence,development,invasion and metastasis of liver cancer[16,17].Similarly,the PI3K/AKT pathway is a key signal transduction pathway that mediates cell growth and prevents cell apoptosis.RTK and JAK,as upstream molecules of the PI3K,play an important role in regulating the PI3K/AKT pathway.It was reported that the PI3K/AKT signaling pathway was abnormally activated in 30% to 50% of patients with HCC patients,and was related to a low survival rate,higher tumor grade,intrahepatic metastasis,vascular invasion and cell proliferation,among other characteristics[18,19].Inhibiting the activation of this pathway can therefore prevent the progression of HCC by inhibiting the survival,migration and invasion of HCC cells.Therefore,inhibiting the activation of the PI3K/AKT signaling pathway has become one of the therapeutic goals for HCC.
The KEGG analysis showed that downregulation of the ALK gene could regulate downstream signal cascade complex systems,including bypassing PI3K/AKT and MAPK,causing a series of changes in cell biology,mediating and regulating cell proliferation,migration and apoptosis[20].The ALK is activated by its endogenous ligands,the heparin-binding cytokine (MK) and pleiotrophin (PTN).These two ligands promote mitosis and angiogenesis in tumor cells.Therefore,the ALK gene has an important role in driving the occurrence and development of tumorsin vivoandin vitro[21].It may be an important target for the anti-liver cancer effects of CSE.
Drug resistance of cells to targeted drugs is currently the main challenge facing the targeted therapy of liver cancer.The main mechanisms of drug resistance include tyrosine kinase mutation,drug efflux,receptor downregulation,and loss of the tyrosine kinase inhibition pathway.Many studies have shown the Axl-mediated drug resistance of various targeted drugs that inhibited Erk,BRAF,PI3Kα,ALK,EGFR or VEGFR,which resulted in a poor prognosis and metastasis[22,23]; The KEGG results also showed that Axl downregulation could regulate the epithelialmesenchymal transition (EMT),consequently inhibiting tumor cell proliferation,differentiation,migration and angiogenesis.
The JAK-STAT signal transduction pathway is an important pathway for the signal transduction of various cytokines and growth factors in mammals; it is also the core pathway in cancer.The pathway comprises non-transmembrane tyrosine kinases JAKs(JAKl,JAK2,JAK3 and TYK2) and STAT proteins(STATl,STAT2,STAT3,STAT4,STAT5a and STAT5b).The activation of this pathway can induce cell proliferation,differentiation,migration and apoptosis[24].Some studies have shown that blocking the JAK/STAT signaling pathway effectively inhibited the proliferation of liver cancer cells and induced apoptosis in liver cancer cells[25].The results of our cell experiments indicated that,compared with the control group,p-STAT3 was significantly downregulated in the CSE group,but that STAT3 protein expression was not significantly downregulated,indicating that CSE could block the activation of STAT3 protein.The expression of VEGF and MMP-2,the downstream regulatory target proteins of STAT3,decreased significantly at a CSE dose of 500 μg/mL.The decreased expression of VEGF and MMP-2 was likely to reduce the proliferation,invasion and metastasis of liver cancer cells.Our results suggested that CSE could inhibit the growth,proliferation,metastasis and invasion of liver cancer cells by inhibiting the phosphorylation of the STAT3 protein and the subsequent reduction in the expression of the downstream target proteins MMP-2 and VEGF.The results of our animal experiments showed that both CSE50and 5-FU could significantly reduce the expression of p-STAT3 and p38MAPK,suggesting that CSE may exert anti-liver cancer effects through regulation of the MAPK and JAK-STAT pathways.The JAK-STAT and Ras/MAPK pathways are the two main pathways of cytokine receptor-mediated intracellular signal transduction,and both interact with different tissues through various kinase-substrate relationships[26].The activation of STAT requires the assistance of cytoplasmic kinases,and p38MAPK is involved in the phosphorylation of its erine site[27].CHUNG et al.[28]observed that MAPK could phosphorylate the STAT3 serine 727 site and activate STAT3.STEPHANOU et al.[29]also found that p38 could directly phosphorylate STAT1 and STAT3 at serine 727 ; therefore,the inhibition of STAT3 phosphorylation may be an important mechanism of the anti-liver cancer activity of CSEin vivo.KEGG pathway analysis showed that downregulation of TYK2 could directly regulate the JAK-STAT signaling pathway.In carcinogenic environments,overactivation of TYK2 led to abnormal activation of STAT1,STAT3 and STAT5[30].The downregulation of TYK2 reduced the invasiveness of tumor cells in mouse models of invasive lymphoma[31].Therefore,blocking the activation of the STAT3 signaling pathway may another key mechanism of the anti-liver cancer activity of CSE.
In conclusion,we confirmed the anti-liver cancer effects of centipede extract,and showed that effects on the JAK-STAT signaling pathway,MAPK signaling pathway,and the PI3K/AKT signaling pathway may be central to these effects.However,downregulation of ALK,which mediates PI3K/AKT and MAPK bypass,and molecules such as TYK2 and AXL,which are closely related to tumor proliferation and drug resistance,may be important targets for the anticancer activity.Further examination in future studies to obtain sufficient evidence,and to provide a strong theoretical basis for the clinical use of centipede extract as a therapy for liver cancer.
Acknowledgements
We thank for the funding support from the National Natural Science Foundation of China (No.81473617),the Science and Technology Department of Hunan Province (No.2017SK50310) and the Hunan Education Department’s Science & Research Project(No.16K066).
Competing Interests
The authors declare no conflict of interest.
杂志排行
Digital Chinese Medicine的其它文章
- Protective Effects of Jin Bai Mei Yan Prescription on Oxidative Damage and Photoaging Induced by Ultraviolet B in HaCaT Cells
- Compound Chai Jin Jie Yu Tablets,Acts as An Antidepressant by Promoting Synaptic Function in the Hippocampal Neurons
- Systematic Pharmacological Strategies to Explore the Regulatory Mechanism of Ma Xing Shi Gan Decoction on COVID-19
- Network Pharmacology Approach to Investigate the Preventive Mechanism of Hunan Expert Group Recommended Chinese Medicine Prevention No.2 Prescription Against COVID-19
