东荣矿区煤样氧化反应动力学热分析
2020-06-16赵维国李经文张辛亥卢苗苗林清松张家亮
赵维国 李经文 张辛亥 卢苗苗 林清松 张家亮

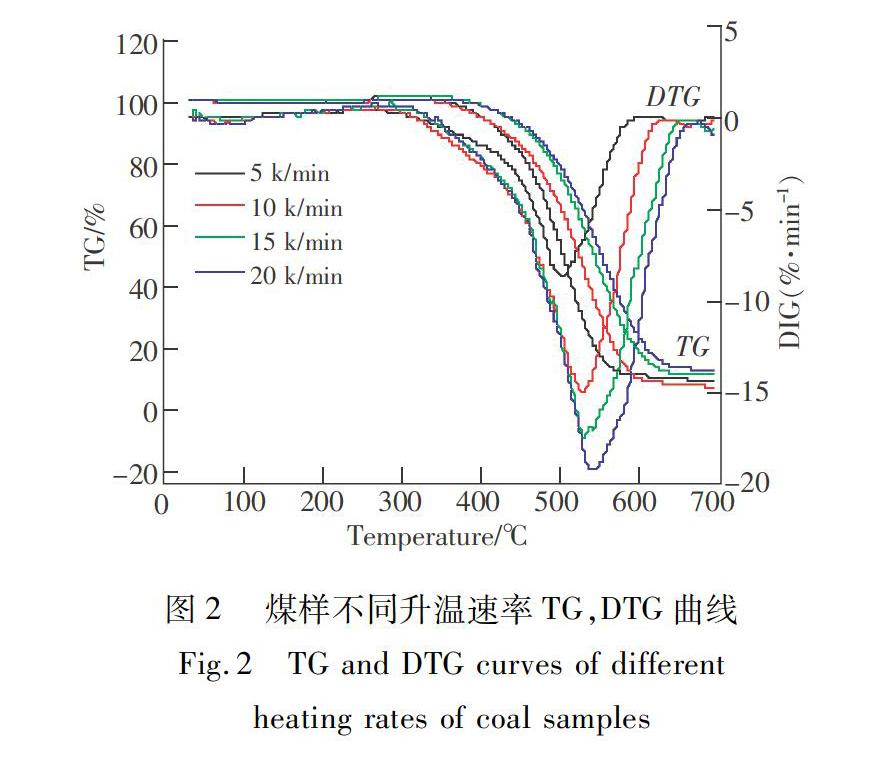

摘 要:東荣矿区煤层自燃现象较为严重,煤的氧化动力学参数是反映煤自燃倾向性的重要指标,为防治东荣矿区煤层自燃,在不同升温速率条件下的基础上,应用热重分析实验,研究了东荣煤样升温氧化过程中质量变化的规律,同时结合15种气固反应机理函数,运用atava法对煤样进行动力学分析,确定了煤氧化机理函数,及活化能、指前因子及反应级数等动力学参数,并应用Ozawa法对得到的动力学参数结果进行了验证。实验表明:不同升温速率下煤样氧化自燃总反应历程相似,热分析曲线的变化规律相同,但随着升温速度的加大,曲线有向右平移的趋势。计算得到煤样高温剧烈氧化时的反应级数为1级,反应动力学模式为一级化学反应,其表观活化能为174.588 kJ/mol,指前因子为5.729×1010.
关键词:热重实验;特征温度;热分析动力学;活化能
中图分类号:TD 752.2
文献标志码:A
文章编号:1672-9315(2020)02-0238-06
DOI:10.13800/j.cnki.xakjdxxb.2020.0207开放科学(资源服务)标识码(OSID):
Kinetics and thermal analysis of oxidation reaction of
coal samples in Dongrong mining area
ZHAO Wei-guo1,LI Jing-wen2,3,ZHANG Xin-hai2,3,
LU Miao-miao2,3,LIN Qing-song4,ZHANG Jia-liang4
(1.Institute of Mining,Liaoning Technology University,Fuxin123000,China;
2.Key Laboratory of Western Mine Exploitation and Hazard Prevention,Ministry of Education,
Xian University of Science and Technology,Xian 710054,China;
3.College of Safety Science and Engineering,Xian University of Science and Technology,Xian710054,China;
4.Inner Mongolia Yitai Coal Co.,Ltd.,Ordos 017000,China)
Abstract:The spontaneous combustion of coal seam in Dongrong mining area is quite serious,and oxidation kinetics parameters of coal are important indicators to the tendency of spontaneous combustion of coal.In order to prevent and control spontaneous combustion of coal seam in Dongrong mining area,the law of mass change in the process of heating and oxidating of Dongrong coal sample was studied by thermogravimetric analysis experiment under different heating rate conditions.
And with 15 kinds of gas-solid reaction mechanism functions and atava in mind,the kinetic analysis of coal samples was carried out,and the mechanism function of coal oxidation and the kinetic parameters such as activation energy,pre-exponential factor and reaction series were determined.The results of the kinetic parameters obtained were verified by Ozawa method.The experimental results indicate that the total reaction process of coal sample oxidation spontaneous combustion is similar under different heating rates,and the change rule of thermal analysis curve is the same,but with the increase of heating rate,the curve has the trend of moving to the right.It is calculated that the reaction order of coal sample is first order and the reaction kinetics model is first order chemical reaction.The apparent activation energy is 174.588 kJ/mol,and the pre-exponential factor is 5.729×1010.
