Al2O3/PVdF-coated Nonwoven Separator for Lithium-ion Battery: Development and Characterization
2019-11-18,,,
, , ,
(Department of Chemistry, Zhejiang University, Hangzhou 310027, China)
【Abstract】 Al2O3/PVdF-coated nonwoven separators were successfully developed by coating a homogeneous slurry with different weight ratios of Al2O3 particles and PVdF binder. The morphology, pore size distribution, thermal stability, wettability, and electrochemical properties of the composite Al2O3/PVdF/nonwoven separators, the nonwoven and commercial Celgard 2300 separator were analyzed. The results show that the CS-5 separator (Al2O3/PVdF (5/5wt%)), compared to the nonwoven, develops a hydrophilic porous structure, the pore size is around 0.1μm, the porosity increases from 68.79% to 75.83%, and the electrolyte uptake increases from 172% to 487%; In comparison with the Celgard 2300 separator, the CS-5 separator exhibits excellent thermal stability with almost no thermal shrinkage at 150℃, and enhanced electrolyte wettability. Additionally, the battery assembled with CS-5 separator exhibits the highest specific capacity of 512mA·g-1 and good cycling performance after 100 charge-discharge cycles at a current density of 100mA·g-1.
【Key words】 separator; lithium-ion battery; thermal stability; electrochemical performance
1 Introduction
The rechargeable lithium-ion batteries (LIBs) have become the key component of the portable electronic devices and hybrid electric vehicles due to their superior energy density and excellent cycle life[1-3]. Despite its impressive growth in application, the performance standards underlying LIB including energy and power densities, cycle lives, and safety concerns still need to be improved[4]. Among the various standards, the safety concerns should be addressed first to enable the process of its broad applications[5]. From the viewpoint of suppressing safety concerns, a separator is considered a critical component to secure the battery safety as its major function is to physically isolate the anode and cathode while enabling free ionic transport and isolating electronic flow[6-8]. At present, microporous polyolefin membranes such as polypropylene (PP), with excellent mechanical strength, chemical stability and low-cost, have been widely applied as batteries separator[9-11]. However, their poor thermal stability and thermal shrinkage have raised serious concerns[12]. They could shrink and even melt in the vigorous conditions such as abnormal heating, which results in physical contact between the cathode and anode and thus a short circuit leading to thermal runway of the battery[6]. Therefore, It is urgently needed to develop anti-shrink and heatproof separators for a safer lithium ion battery.
Among the many alternatives, the use of nonwovens has drawn great attention[13-17]. Through the selection of the raw materials, the thermal stability of the nonwoven could be well improved, which can reduce the possibility of separator meltdown under exposure to heat generated by overcharging or internal short-circuiting[18]. On the other hand, nonwoven separators are featured by high porosity, which could efficiently improve the electrolyte absorption and ionic conduction[13]. However, their large pore size and broad pore-size distribution could lead to poor battery performance, which hinder their application to the LIB[19]. One possibility to address these drawbacks of nonwoven separator is the incorporation of inorganic powders such as silica or alumina[15, 20-22]. Another strategy is to coat polymers on the surface of the base nonwoven by the method of dip-coating process[23]or phase inversion[13].
In this work, we report the fabrication of a new composite separator by a facile dip-coating process, which comprises poly(vinylidene fluoride) (PVdF), alumina and nonwoven support. The nonwoven consisting of natural (celluloses) and synthetic (poly(ethylene terephthalate) (PET)) fibers acted as a mechanical support that contributes to improving the thermal shrinkage of the composite separator. The alumina powders were interconnected by the PVdF binder and served as a pore size controller for the composite separator. A homogeneous and labyrinth-like surface structure was generated by controlling the content of the coating solution. The Al2O3/PVdF/nonwoven composite separators were tested over their physical, thermal, and electrochemical properties. Compared to the commercial separator (Celgard 2300), the composite separators exhibited higher porosity and superior dimensional thermal stability, indicating they could tremendously optimized the electrochemical performance and enhanced the safety of the LIBs. Herein, it could be demonstrated that the Al2O3/PVdF/nonwoven composite separators would hold considerable potential in the development of separators for high-performance lithium-ion batteries aiming at future applications.
2 Experiments
2.1 Preparation of the composite separators
A thin nonwoven membrane (average thickness=17μm) manufactured by bonding numerous celluloses and PET fibers together through a papermaking method was chosen as the coating substrate. Poly(vinylidene fluoride) (PVdF) was used as polymer blinder. The Al2O3powder (average particle size=500nm) was first dried at 105℃ for 2h. After that, the PVdF and Al2O3powders were dissolved in the solvent of 1-methyl-2-pyrrolidone (NMP) at room temperature, the mixed solution was subsequently dispersed for 30 min in an ultrasonic apparatus and further blended by magnetic stirring for 12h. Next, the nonwoven was immersed into the prepared coating solution for 30s, and followed by a simple dip coating to secure uniform thickness. After the coating process, the coating solution-immersed separators were dried at room temperature and further dried in a vacuum oven at 80℃ for 12h. The composite separator with the weight ratios of the Al2O3/PVdF mixture controlled in 1/9, 2/8, 5/5 were respectively prepared and referred to as CS-1, CS-2 and CS-5 separator. In the meanwhile, a commercial separator (Celgard 2300, thickness=25μm) severed as the control sample.
2.2 Physical and electrochemical characterizations
The morphologies of the composite separators and commercial Celgard 2300 separator were observed by scanning electron microscope (SEM SU-8010). The pore-size distribution of the base nonwoven membrane and the composite Al2O3/PVdF/nonwoven separators were measured by the method of mercury intrusion using Auto Pore IV 9500 (Micro-meritics).
For comparing the thermal stability of the composite separators and Celgard 2300 separator, all separators were subjected to a hot oven at 150℃ for 30min. The thermal shrinkage was determined by measuring the dimensional change (area-based) before and after the heat treatment. To further study the shutdown behavior of the separators, thermal gravimetric analyzer (TG) measurements were performed with the temperature ranging from room temperature to 700℃ at a heating rate of 10℃ per minute in the nitrogen atmosphere.
To evaluate the electrochemical performance of the composite separators and the Celgard 2300 separator, standard CR 2025 type coin batteries were assembled, using 1 M LiPF6-EC/DMC (1∶1 in volume) as electro-lyte, Lithium-foil as counter/reference electrode, the working electrode was made by the following steps. A homogeneous slurry was prepared by mixing specific material (silicon/carbon)[25]with acetylene carbon black and PVdF with a weight ratio of 70∶15∶15 and using NMP as solvent; The slurry was spread onto copper foil and dried in a vacuum oven at 80℃ for 12 h and then pressed at a pressure of 10 atm; All batteries were assembled in an argon-filled glove box with the water and oxygen contents less than 0.1ppm. The cyclic perfor-mance (galvanostatic charge-discharge) was carried out at between the cut-off voltage of 0 and 3V at a current density of 100mA· g-1, and the rate discharge performance was tested at various current densities of 100, 200, 500, 1000, 2000 and 3000mA· g-1. The specific capacities were based on the mass of the working composite electrode. The electrochemical impedance spectroscopy (EIS) was carried out using a CHI760D electrochemical work station system (CH instruments) with a scan frequency ranged from 0.01Hz to 1000kHz at amplitude of 10mV.
3 Results and discussion
3.1 Physical characterization
The thickness and weight of the separators before and after thedip-coating process were measured and shown in Table 1. The thicknesses of the composite separators are obviously thinner than the Celgard 2300 separator (25μm). Thinner separator can provide much more space for active materials, thus increases the efficiency of the battery[24]as well as lowers the resistance of the separator[25]. With increasing the PVdF content of the coating layer, the thickness slightly increases from 17.0μm (the pristine nonwoven membrane) to 18.8μm (the CS-5 separator). In addition, the weight of the Al2O3/PVdF/nonwoven composite separators increase from 13.76g·m-2(the pristine base nonwoven membrane) to 21.16g·m-2(the CS-1 separator). Both changes mentioned above corroborate that the coating layer is deposited on the surface of the nonwoven membrane.
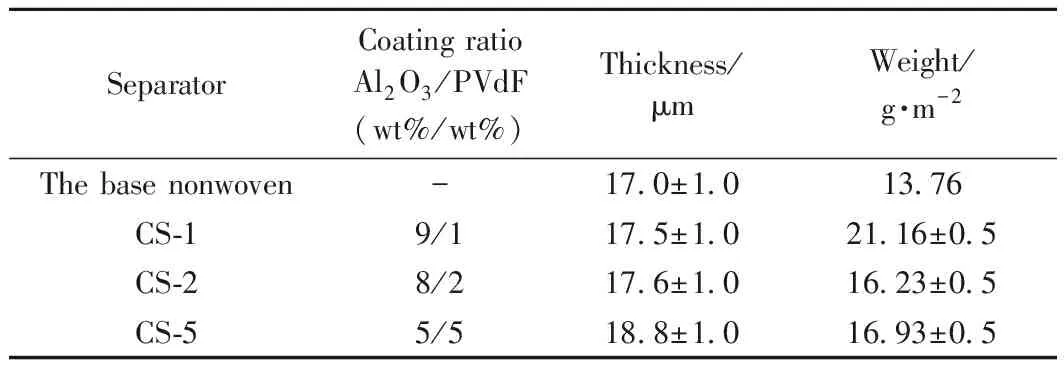
Table 1 Weight and thickness of the separators before and after coating
The above result has been also supported by the top surface SEM analysis. The pristine base nonwoven comprises of two different scale fibers and exhibits obvious multi-fibrous structure (Fig.1 (a)). Among the multi-fibrous layers, there are lots of big pores which are not appreciated with the lithium-ion batteries, because it might trigger the internal short. Thus, in the preparation of the composite separator, the Al2O3powder was added for filling these big pores and the PVdF played as binder to further optimize the effect of filling. As the content of the PVdF binder in the coating layer increased from 10 wt% to 50 wt%, the pristine base nonwoven surface began to generate a new film (Fig.1 (b~d)). The base nonwoven is not completely covered by the thin film of the CS-1 separator (Fig.1 (b)), and the big scale fibers of the nonwoven can still be clearly seen. Fig.1(c) shows the surface image of the CS-2 separator which is much more covered by the new generated film compared to the CS-1 separator. Differently, the morphology of the CS-5 separator (Fig.1(d)) with the highest amount of PVdF is mostly covered with a thin film formed by PVdF. The morphology of the PVdF film is similar to a network structure with lots of micron elliptic pores. Fig.1 (e) presents the SEM image of those micron pores at higher magnification, which shows that there are many nano-sized Al2O3particles in the micron elliptic pore. This indicates that those existences of the big pores do not lead to a problem of pore size while develop a porous structure. Fig.1 (f) presents the cross-section of the CS-5 separator, which shows that the gaps of the base nonwoven are filled by the mixture of Al2O3and PVdF. This indicates that the Al2O3and PVdF infiltrate into the base nonwoven during the coating process.
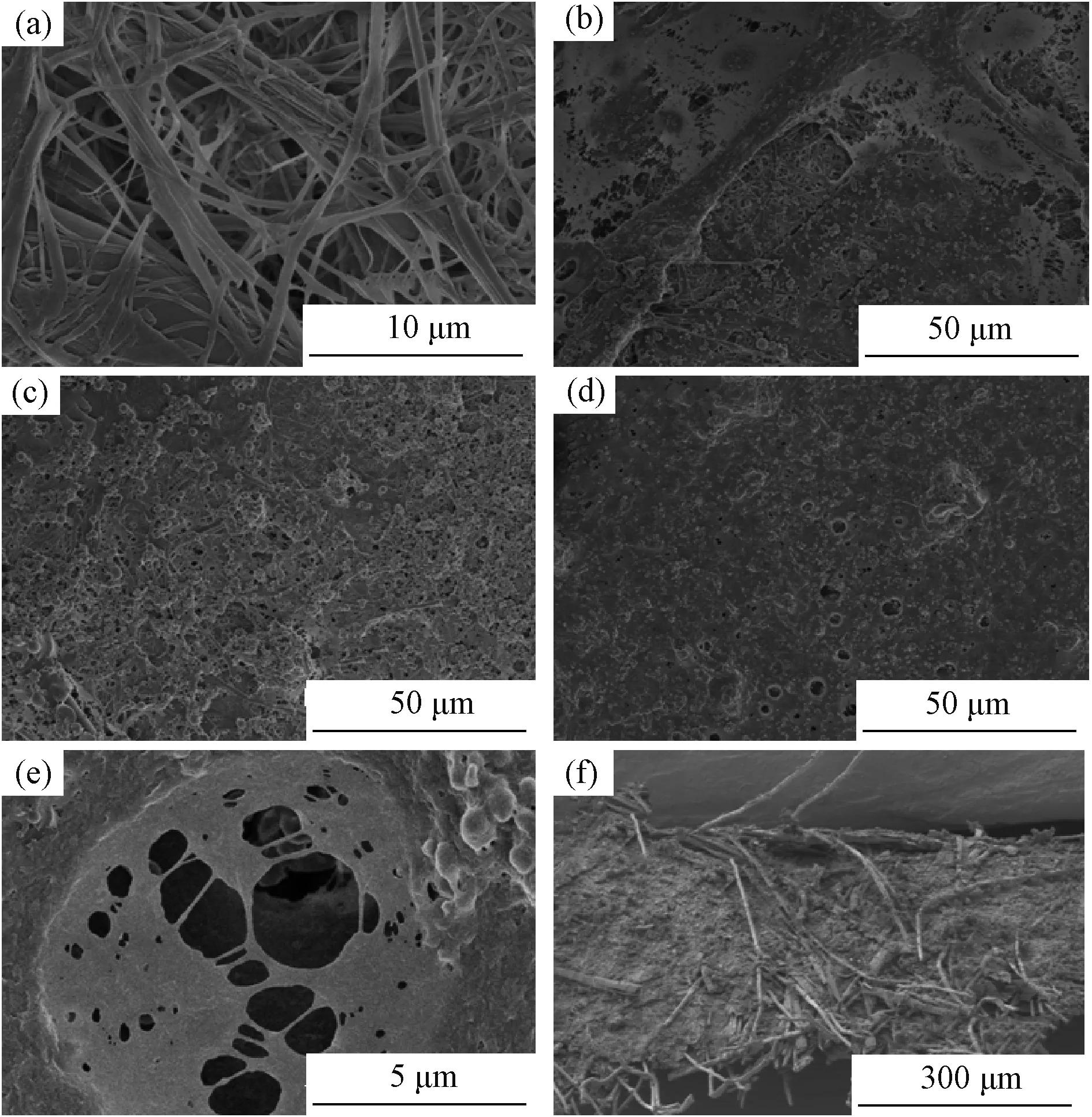
Fig.1 SEM images: (a) the base nonwoven; (b) CS-1 separator; (c) CS-2 separator; (d), (e) the images of the CS-5 separator with different magnifications; (f) cross-section of the CS-5 separator
Large pore size and broad pore-size distribution of separators will lead to a poor cycle life of the battery. Therefore, it is urgent to further investigate the pore size and distribution of the separators. Fig.2 shows pore-size distributions of the base nonwoven and composite Al2O3/PVdF/nonwoven separators measured by using mercury porosimeter. The peaks of the pore-size distribution curve represent the main pore size of the separator. It is observed that there are many small and wide peaks exhibited in the base nonwoven membrane. Among those peaks, there are two relatively higher peaks at about 0.1 and 10μm. This indicates that the base nonwoven separator has a wide pore-size distribution and the main pore sizes focus on 0.1 and 10μm which is incapable to use for battery. With increasing the weight ratios of Al2O3/PVdF from 9/1 to 5/5, the peak at the 0.1μm quickly rises and the peak at 10μm gradually falls, indicating that the pore size decreases at 10μm but increases at 0.1μm. In case of the CS-5 separator, a single large and sharp peak at 0.1μm is present in the pore-size distribution curve, indicating that CS-5 separator has much more homogeneous and narrow pore size and distribution compared to the base nonwoven. The improvement in pore size and distribution of the CS-5 separator might greatly contribute to the new generated PVdF film on the base nonwoven.
Those results are consistent with the value of mean pore size as shown in the Table 2. The CS-5 separator observes to have higher porosity of 75.83% compared to the value of other samples and the Celgard 2300 separator (51.40%), correspondingly higher electrolyte uptake of 487% (Celgard 2300 separator: 172%). Obviously, the new generated porous structures of the composite separators enable the electrolyte to reach the interior which reasonably increase the value of porosity. Higher porosity provides a better reservoir for the electrolyte in the cell[26]and leads to a higher electrolyte uptake value. The separator with high porosity and electrolyte uptake brings advantages to the battery performance such as high energy and power densities[6]. Therefore, the CS-5 separator with thin thickness, high porosity and electrolyte uptake has relatively better physical performance than Celgard 2300 separator.
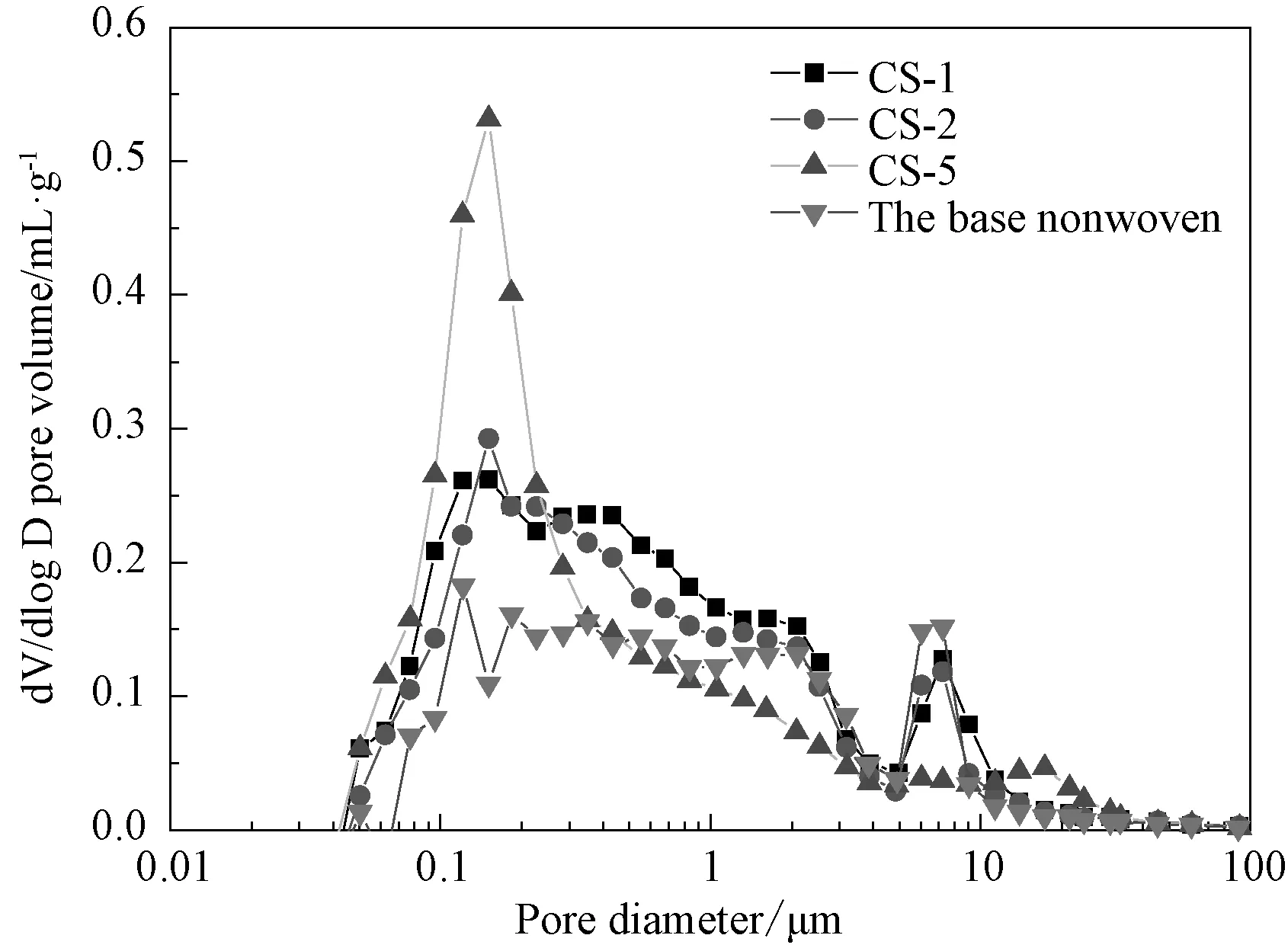
Fig.2 Pore-size distribution curves of the base nonwoven, CS-1, CS-2, and CS-5 separators
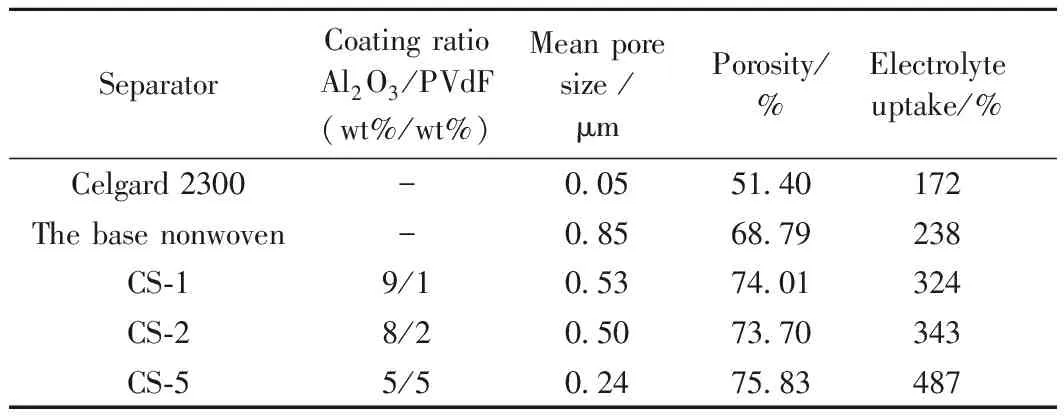
SeparatorCoating ratio Al2O3/PVdF(wt%/wt%)Mean pore size /μmPorosity/%Electrolyte uptake/%Celgard 2300-0.0551.40172The base nonwoven-0.8568.79238CS-19/10.5374.01324CS-28/20.5073.70343CS-55/50.2475.83487
3.2 Thermal characterization
In the process of charging and discharging, the battery will release a great deal of heat, which can result in deformation of separator and lead to an internal short circuit of the battery. Hence the thermal stability of the commercial Celgard 2300 separator and composite Al2O3/PVdF/nonwoven separators were investigated by exposing them to 150℃ for 30 min. As presented in Fig.3, the Celgard 2300 separator (Fig.3(a)) presents a high degree of shrinkage of about 35% in length (about 30% in area) after the high-temperature exposure process. In contrast, the composite Al2O3/PVdF/nonwoven separators (Fig.3(b)~(d)) show almost no thermal shrinkage at all because the base nonwoven which is made by plenty of high melting PET fibers provides enough support to avoid the thermal deformation at high temperature. Hence, the risk probability of a short circuit occurring could be significantly reduced by using the composite Al2O3/PVdF/nonwoven separators.
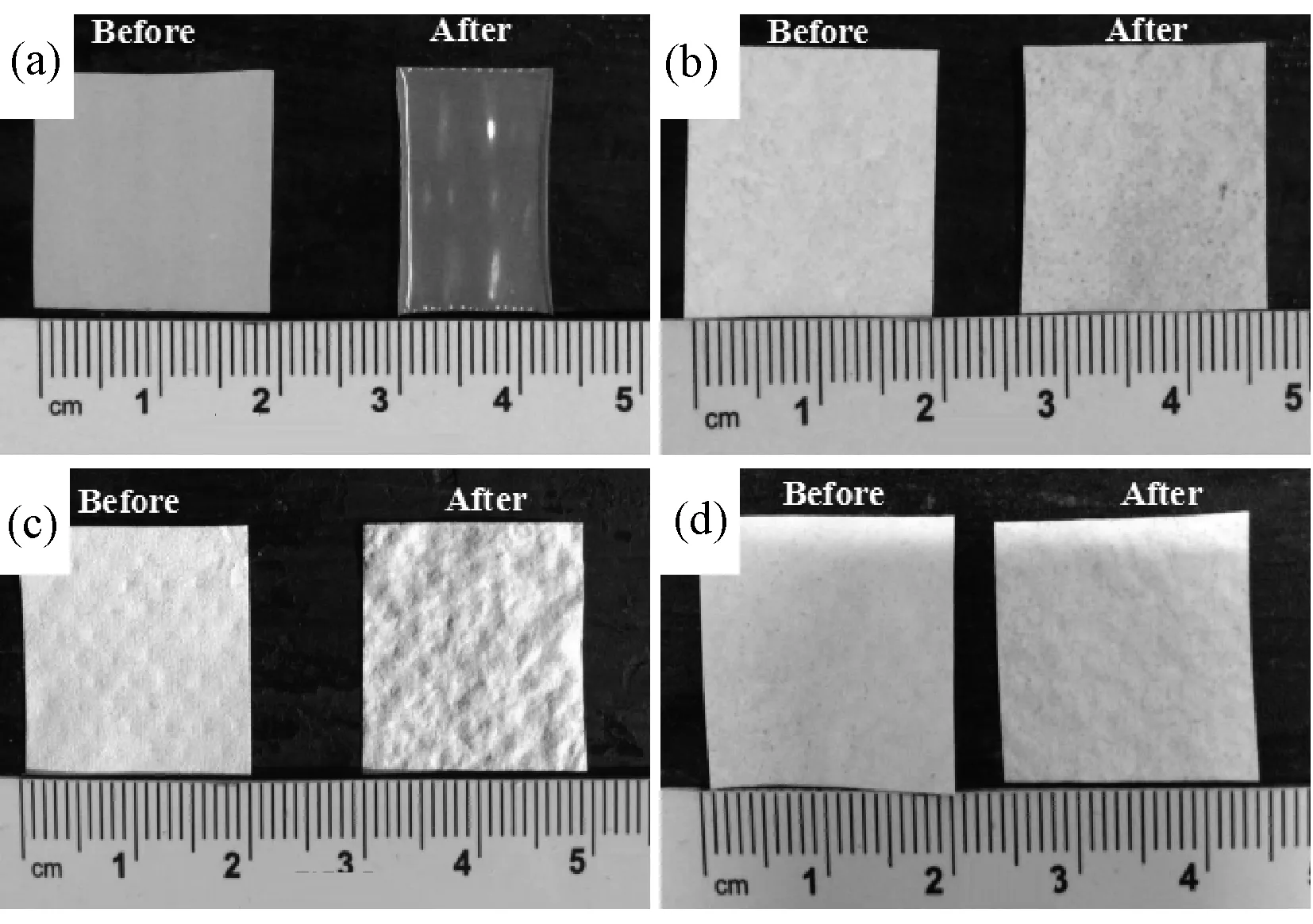
Fig.3 Photographs of the Celgard 2300 separator (a) and the composite Al2O3/PVdF/nonwoven separator s before and after hot oven test at 150℃ for 30min (b: CS-1 separator; c: CS-2 separator; d: CS-5 separator)
The TG test was further carried out for investigating the thermal stability of the base nonwoven and the composite Al2O3/PVdF/nonwoven separators. As shown in the Fig.4, the composite separators present better thermal performance with the increase of the weight ratio of PVdF and Al2O3. Moreover, the base nonwoven and the CS-5 separator lose about 7% of weight at 277℃ and 309℃, respectively, indicating that the initial decomposition temperature for the CS-5 separator mildly shifts to a higher value when a PVdF film was generated on the base nonwoven. Those results indicate that the composite Al2O3/PVdF/nonwoven separators have excellent thermal stability especially the CS-5 separator.
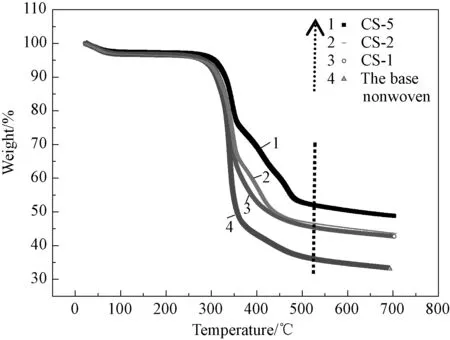
Fig.4 TG curves of the base separator and the composite Al2O3/PVdF/nonwoven separators
3.3 Wettability characterization
The wetting behavior of the commercial Celgard 2300 separator and the composite separators were investigated by dropping liquid electrolyte (1 M LiPF6-EC/DMC (1∶1 in volume)) onto their surfaces and observing the surface image of the separators. As shown in Fig.5a, an isolated liquid droplet can be observed on the surface of the Celgard 2300 separator, indicating that it is not completely wetted by the liquid electrolyte. Conversely, the composite Al2O3/PVdF/nonwoven separators are quickly and entirely wetted by the liquid electrolyte, indicating the wettability of the composite separators were better than that of the Celgard 2300 separator.
To quantitatively evaluate the wetting properties of separators, the contact angles of the Celgard 2300 separator and the composite Al2O3/PVdF/nonwoven separators with liquid electrolyte were measured as shown in Fig.6. The contact angles of dropping the liquid electrolyte immediately are listed in the Fig.6(a)~(d). The Celgard 2300 separator exhibits a contact angle of 49.1° which was much larger than that of composite separators (CS-1:13.8°; CS-2:15.3°; CS-5:27.1°). After 10 s standing, the contact angles of those samples are list in Fig.6(e)~(h). The Celgard 2300 separator remains the same angle of 49.1° after 10 s standing (Fig.6(e)). Moreover, the contact angles of the composite separators decrease to about 8° (CS-1:8.0°; CS-2:7.5°; CS-5:7.2°), indicating that the electrolyte droplets quickly spread into the pores of the composite separators in the 10 s. The interesting observation suggests that the composite Al2O3/PVdF/nonwoven separators are much more hydrophilic to the electrolyte than the Celgard 2300 separator owing to their porous structures.
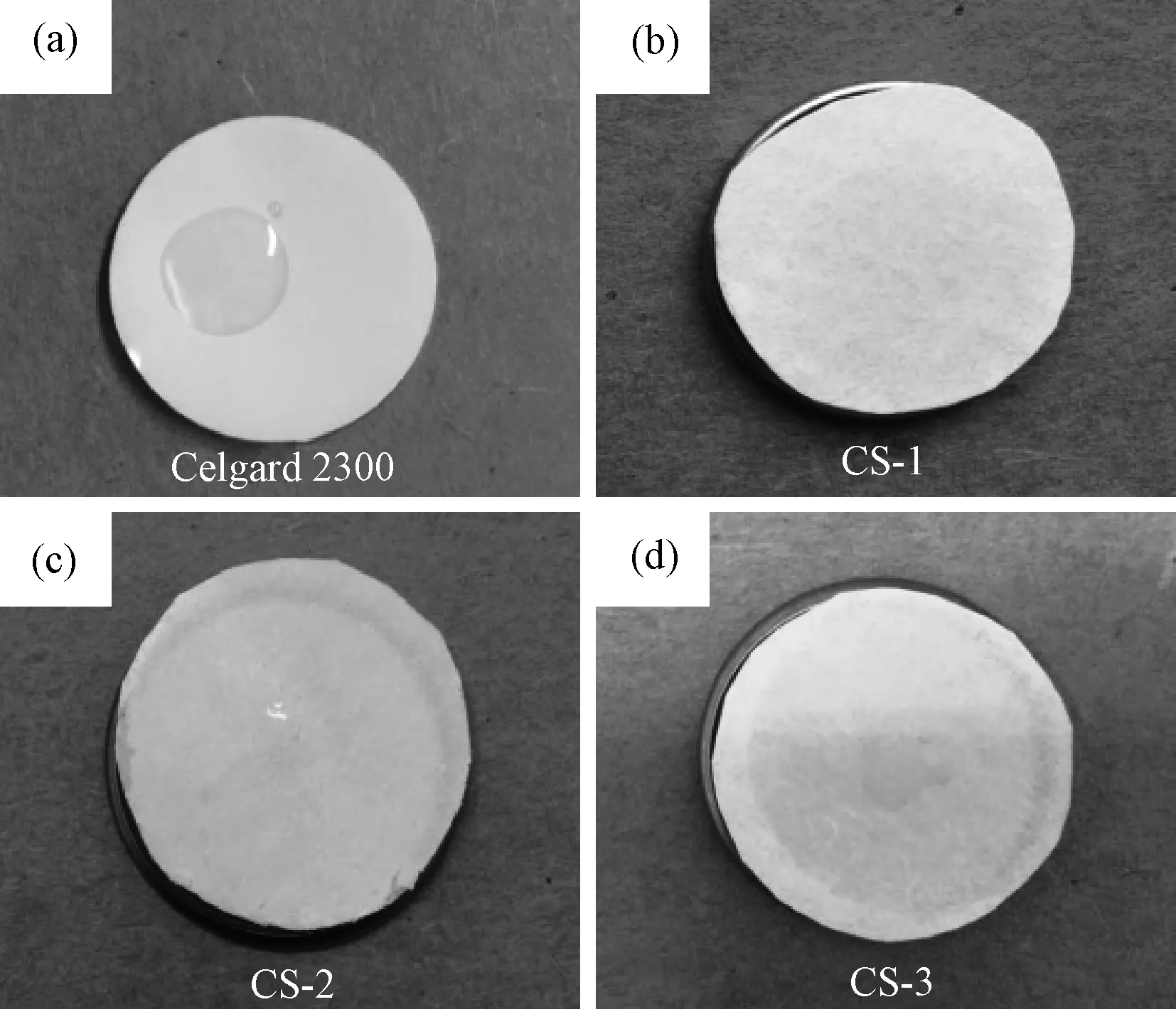
Fig.5 Photographs of the separators wetted by electrolyte (a) the Celgard 2300 separator; (b): CS-1 separator; (c): CS-2 separator; (d): CS-5 separator
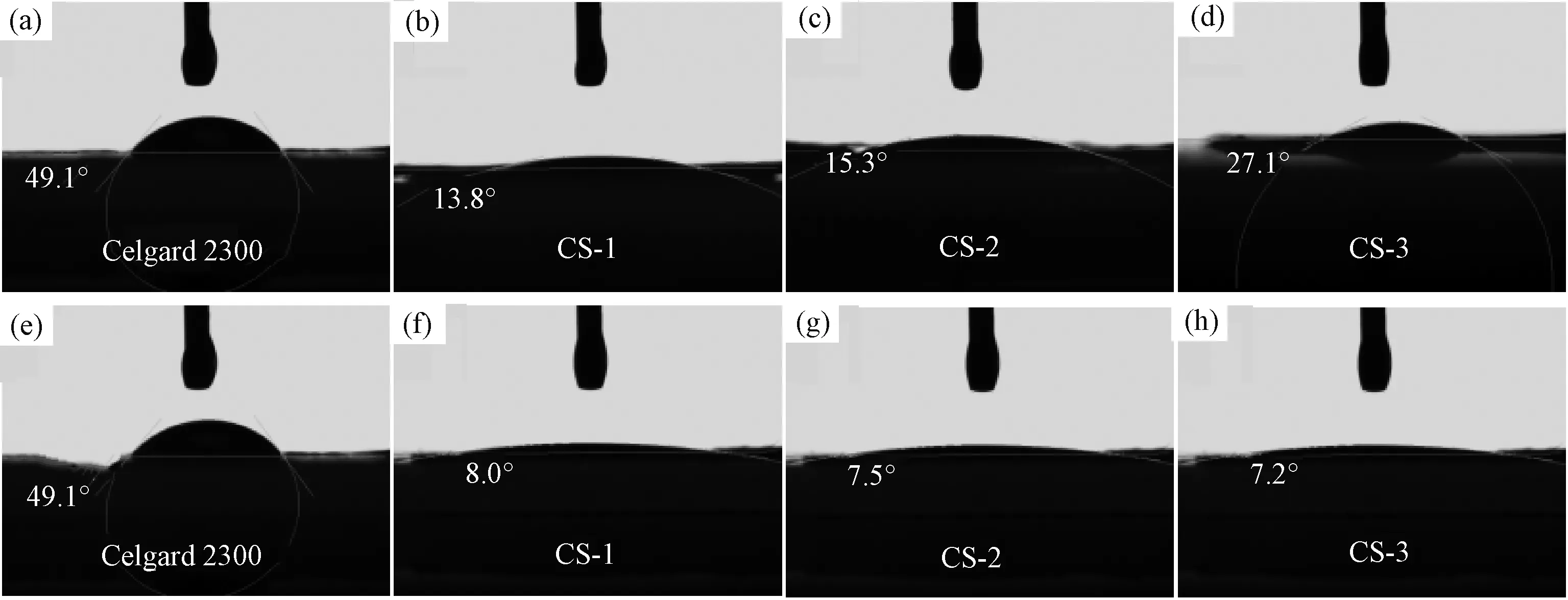
Fig.6 Contact angles of dropping liquid electrolyte immediately (a-d) and after 10 s standing (e-h): (a), (e): the Celgard 2300 separator; (b), (f): the CS-1 separator; (c), (g): the CS-2 separator; (d), (f): the CS-5 separator
3.4 Electrochemical characterization
Fig.7(a) represents the galvanostatic cycling performance of the Celgard 2300 separator, the base nonwoven and the composite Al2O3/PVdF/nonwoven separators at a current density of 100mA·g-1. The discharge capacities of all batteries decrease slowly as the cycle number increases, exhibiting normal cycling performance. Meanwhile, the discharge capacities of the composite separators (especially the CS-5 separator) are significantly higher than that of the base nonwoven separator, which means the composite separators exhibit better electrochemical performance than the base nonwoven. The discharge capacity of the CS-5 separator decreases within the first 20 cycles, then reaches a relatively stable value with the discharge capacity of generally 500mA·g-1. After 100 charge/discharge processes, the discharge capacity is 512mA·g-1. This value is much higher than that of the Celgard 2300 separator (174mA·g-1) and that of other composite separators (the CS-1 separator: 343mA·g-1; the CS-2 separator: 401mA·g-1). The noticeable improvement in the cyclability of the CS-5 separator could be attributed to its high porous structure and excellent wettability. Fig.7(b) summarizes the discharge capacities of the separators as a function of different discharge current densities (from 100 to 3000mA·g-1), it is seen that the capacity gradually decreases along with the increase of the current density. The composite Al2O3/PVdF/nonwoven separ-ators, especially the CS-5 separator (Fig.7(b), blue curve), exhibits excellent discharge capacity in different discharge current density, which appears to higher than that of the Celgard 2300 separator (Fig.7(b), pink curve) and the other separators. At the high current density of 3000mA·g-1, the capacity value of the CS-5 separator decreases to 246mAh·g-1which is still much higher than that of the Celgard 2300 separator (40mAh·g-1). Consistent with the observation on the cyclability, this indicates that the CS-5 separator presents better electrochemical performances than the commercialized Celgard 2300 separators.
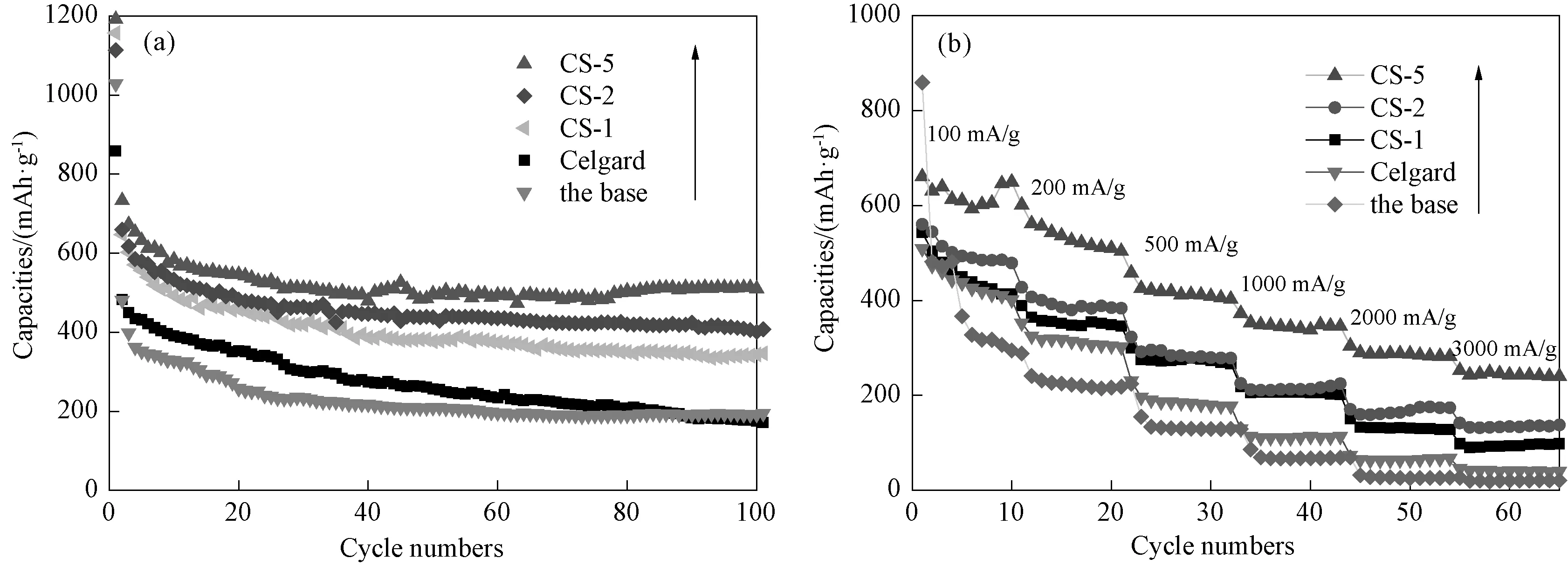
Fig.7 Electrochemical performance of the half batteries assembled with the composite Al2O3/PVdF/nonwoven separators, the base nonwwoven and the Celgard 2300 separator. (a) The discharge cycling performance of the composite separators, the base nonwwoven and the Celgard 2300 separator at a current density of 100mA·g-1; (b) The rate capabilities of the composite separators, the base nonwwoven and the Celgard 2300 separator at various current densities
In order to confirm the compatibility of the battery electrodes with the Celgard 2300 separator and the CS-5 separator, the EISs of two half batteries after 5th and 30th cycle were measured and shown in Fig.8. The increase in the radius of the semicircle after cycling represents the increase in cell impedance which has a negative influence on capacity of cells[27-28]. It should be noted that the increase in cell impedance after 30th cycling is suppressed in the battery assembled with the CS-5 separator. Therefore, the CS-5 separator seems conducive to the interfacial stability of the battery, in accordance with the cycling and rate performance results shown in Fig.7. This may be ascribed to its unique porous structure which improves the intimate contact with the liquid electrolytes. Hence the CS-5 separator is suggested to be used in lithium-ion batteries for better performance.

Fig.8 EIS of half cells after 5th and 30th cycle:(a) the Celgard 2300 separator; (b) the CS-5 separator.
4 Conclusion
A new composite Al2O3/PVdF/nonwoven separator with excellent thermal stability, improved wettability and electrochemical performances was successfully fabricated to meet the requirement for high-safety LIBs. Compared to the commercial Celgard 2300 separator, the CS-5 (Al2O3/PVdF (5/5 wt%)) separator displayed superior dimensional thermal stability (almost no thermal shrinkage at 150℃ for 30 min), indicating that it could largely enhance the safety of the Lithium ion batteries. Furthermore, due to the high porosity (75.83%), enhanced surface wettability and electrolyte uptake of the CS-5 separator, it also showed improved electrochemical performances in terms of cyclability, rate capability and cell impendence.
