Screening the RFX6-DNA binding domain for potential genetic variants in patients with type 2 diabetes
2019-04-16IsmailMahmoudAyatHomsiHamzehAlAmeerJihadAlzyoudMaisDarrasMohammadAlShhabMalekZihlifMamonHatmalWalhanAlshaer
Ismail S Mahmoud,Ayat Homsi,Hamzeh J Al-Ameer,Jihad Alzyoud,Mais Darras,Mohammad Al Shhab,Malek Zihlif,Ma’mon M Hatmal,Walhan Alshaer
Ismail S Mahmoud,Jihad Alzyoud,Mais Darras,Ma’mon M Hatmal,Department of Medical Laboratory Sciences,Faculty of Allied Health Sciences,The Hashemite University,Zarqa 13133,Jordan
Ayat Homsi,Walhan Alshaer,Cell Therapy Centre,The University of Jordan,Amman 11942,Jordan
Hamzeh J Al-Ameer,Mohammad Al Shhab,Malek Zihlif,Department of Pharmacology,Faculty of Medicine,The University of Jordan,Amman 11942,Jordan
Abstract
Key words:Regulatory factor X6;Genetic variant;Diabetes;DNA binding domain
INTRODUCTION
Diabetes mellitus is a group of glucose metabolism disorders characterized by high levels of blood glucose.The disease affects millions of people worldwide,which is usually associated with serious complications that affect various systems of human body[1].Type 2 diabetes (T2D) is mainly manifested by low insulin production by pancreatic cells and/or the produced insulin does not function effectively[2].Alterations in both beta cells average mass and function have been observed and reported in people with diabetes[3-5].
Regulatory factor X (RFX) proteins constitute a family of DNA binding proteins that is conserved in the eukaryotic kingdom[6].In humans,RFXs act as regulatory transcription factors that bind to a conservedcis-regulatory element called the X-box motif,which is typically 14-mer DNA sequences located in specific promoter regions of the genome[7].The function of mammalian RFX proteins has only recently started to emerge,and has shown to play an important role in regulating growth and development,immune response and endocrine secretions[7].The RFX family has seven members of RFX1-7 in mammals,which have wide expression in various tissues and organs.RFX6 is a main member of RFX family that is predominantly expressed in pancreatic islets and encoded by a gene on chromosome 6[7].RFX6 possesses a highly conserved DNA binding domain which is critical for binding of RFX6 to X-box promoter motifs and thus regulating their function.Recent studies conducted in mice demonstrated that RFX6 is specifically required for pancreatic beta cells differentiation during embryonic development[8].Moreover,RFX6 was shown to be an important factor to maintain key features of functionality of mature beta cells,andRFX6gene deletion in adult mice beta cells was shown to disrupt glucose homeostasis and caused glucose intolerance,impaired beta cell glucose sensing and defective insulin secretion[9].
In 2017,Varshneyet al[10]published an interesting study in the Proceedings of the National Academy of Sciences,where they performed an integrated analysis of molecular profiling data of the genomic DNA,epigenome and transcriptome in diabetic pancreatic beta islets,to understand the potential connections between genetic variants,chromatin landscape,and gene expression in T2D.The study showed that most of the reported genetic variants in T2D are enriched in regions of the DNA where RFX transcription factors are predicted to bind.The study also concluded that these genetic variants that increased the risk of T2D are predicted to disrupt mainly the binding of RFX6 to genomic DNA[10],indicating that RFX6 binding to X-box promoter motifs could be disrupted in T2D.
In this study,we sought to investigate if any structural genetic defects could be present in the RFX6-DNA binding domain in T2D patients that could potentially inhibit its function in diabetes.
MATERIALS AND METHODS
Patient and control samples
Initially,a total of 98 blood samples (49 samples from T2D patients,49 from healthy volunteers (control group)) were collected from Jordanian population (Table1).The study was then extended to investigate the association between the identified intronic variant (IVS6+31 C>T) and diabetes.A total of 283 blood samples (141 from T2D patients,142 from healthy volunteers) were included in the extended study (Table1).Diabetic participants who enrolled in this study were Jordanian adults (age ≥ 20 years),including both females and males,with known history of diabetes and recruited from Jordanian medical centres during the time period between Dec 2015 and July 2017.Controls were unrelated to diabetic patients and had no history of diabetes,as determined by history and lab examination.All blood samples were collected according to protocols approved by the Institutional Review Board,and informed consents were obtained from participants included in the study.
Biochemical examination
The levels of blood glucose and glycosylated Hb (HbA1c) were evaluated in the participants of this study.Blood glucose level was measured by the glucose oxidase method using Cobas c111 analyzer (Switzerland).The percentage of HbA1c in blood was determined using ion-exchange high-performance liquid chromatography (D-10™ Bio Rad,United States).
Molecular techniques
For molecular assays,DNA was extracted from venous whole-blood samples,and then collected in EDTA containing tubes,using Wizard genomic DNA purification kit(Promega,United States).Extracted DNA was stored at -20°C.Polymerase chain reaction (PCR) was used to amplify genomic DNA encompassing the coding sequences and intronic borders of exons 3,4,5 and 6 of theRFX6gene (NCBI:NG_027699.1).The primers used for PCR assay were as described in (Table2).The PCR amplification reactions were performed in a total volume of 25 μL in 0.2 mL PCR tubes containing 200 ng of genomic DNA,5 μL of 5× FIREPol®Master Mix (Solis BioDyne) with 7.5 mmol MgCl2and 10 pmol of each primer (Gene Link,United States).All PCR reactions were performed using C1000 Touch™ Thermal Cycler (Bio-Rad;United Kingdom) and the reaction conditions were as follows:initial denaturation of 5 min at 95°C,followed by 29 cycles of 30 s at 95°C,30 s at specific annealing temperature (Table2),1 min at 72°C and a final extension of 6 min at 72°C.All PCR products were checked by gel electrophoresis to verify correct product size,then purified and sequenced using the same forward primer used for the gene amplification.DNA sequencing reactions were performed at Macrogen Inc.,South Korea,using BigDye(R) Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems,United States) and the ABI PRISM 3730XL analyzer (Applied Biosystem,United States).
Statistics
Data analyses were performed using IBM Statistical Package for the Social Sciences software (SPSS,version 19).Pearson’s chi-squared or Fisher’s exact tests were used to test an association between categorical variables (i.e.,gender and genetic variant) and case-control status.Student t-test was used to assess the significance of difference of means of age,blood glucose and glycosylated Hb between case and control groups.A 95% confidence interval for the odds ratio was calculated and used to describe the results.For sample size calculation,the Cochran’s formula was used[11].
RESULTS
In this study,the screening for structural genetic variants in specific regions of RFX6 gene was carried out in T2D patients in attempt to discover new potential mutations that could mediate the pathogenesis of T2D.Evaluation of levels of blood glucose and HbA1c of enrolled subjects was performed to discriminate between diabetic and nondiabetic (Table1).The DNA sequence analysis of exons 3,4,5 and 6 ofRFX6gene in 49 diabetic patients revealed the absence of any genetic mutation.However,the DNA sequencing of introns borders revealed the presence of a heterozygous genetic variant(IVS6+31 C>T) within the intron 6 ofRFX6gene,where C is substituted by T,31 nucleotides downstream of the end of exon 6 (Figure1).To investigate the significance of the identified intronic variant in T2D,the study was extended and the IVS6+31 C>T was screened in 283 samples (141 diabetic and 142 healthy controls).DNA sequence analysis of the samples revealed the identification of the heterozygous IVS6+31 C>T in 9.2% and 8.4% of diabetic and control groups respectively,with no significant association in genotype or allele frequency between diabetic and control groups (Table3).

Table1 Demographic and clinical characteristics of diabetic patients and healthy controls
The demographic characteristics of the study population (age and gender) are shown in Table1.In primary screening study,there was no statistically significant difference in male/female proportion (P= 0.285 andP= 0.217,respectively) or age of gender (P= 0.149 andP= 0.065,respectively) between case and control groups.As for the extended screening study of IVS6+31 C>T variant,no statistically significant difference in male/female proportion was found between case and control groups (P= 0.105 andP= 0.136,respectively).However,there was a significant difference in age of male/female of case-control status (P= 0.001 andP= 0.024,respectively).
DISCUSSION
Recently,it has been proposed that a loss of pancreatic beta-cells mass,differentiation and function is a hallmark of T2D[1,2].Concurrently,the regulation of beta cells growth and differentiation has been under intensive investigation,and several lines of evidences have indicated the role of key transcription factors in controlling the function state of beta cells.For example,evidences coming from loss-of-function studies in adult mice beta cells have revealed that transcription factors such as NeuroD1[12],Nkx6.1[13]and Pdx1[14]are important in maintaining the differentiation and function state of pancreatic beta cells.Thus,it appears that loss of function of key beta cell transcription factors results in the loss of both beta cell identity and function.More recently,RFX6 transcription factor has been shown to play a key role in regulating the state of pancreatic beta cells differentiation and function[9].
RFX6 contains a highly conserved DNA binding domain that facilitates their binding to X-box promoter motif of certain genes,which is essential to regulate the transcription ofRFX6-target genes[7].It has been shown that genetic alterations in the RFX6-DNA binding domain could be associated with neonatal diabetes.In fact,mutations in the RFX6-DNA binding domain are assumed to be the cause of neonatal diabetes in Mitchell-Riley syndrome,through the production of a defective RFX6 protein[15].In this project,we sought to detect if any genetic mutation could be present in the RFX6-DNA binding domain in T2D.Based on our findings we conclude that structural mutations in the DNA binding domain of RFX6 are unlikely to exist in T2D.However,another large-scale study could increase the statistical power of our results.In addition,it is noteworthy to mention that RFX6 proteins contain other conservedregions including B,C,and D domains[6].These domains are thought to be involved in RFX6 oligomerization which are required for DNA binding and activation[7].Therefore,screening the other functional domains of RFX6 may provide more insights into the potential mechanism by which RFX6 binding to DNA is abrogated in diabetes.
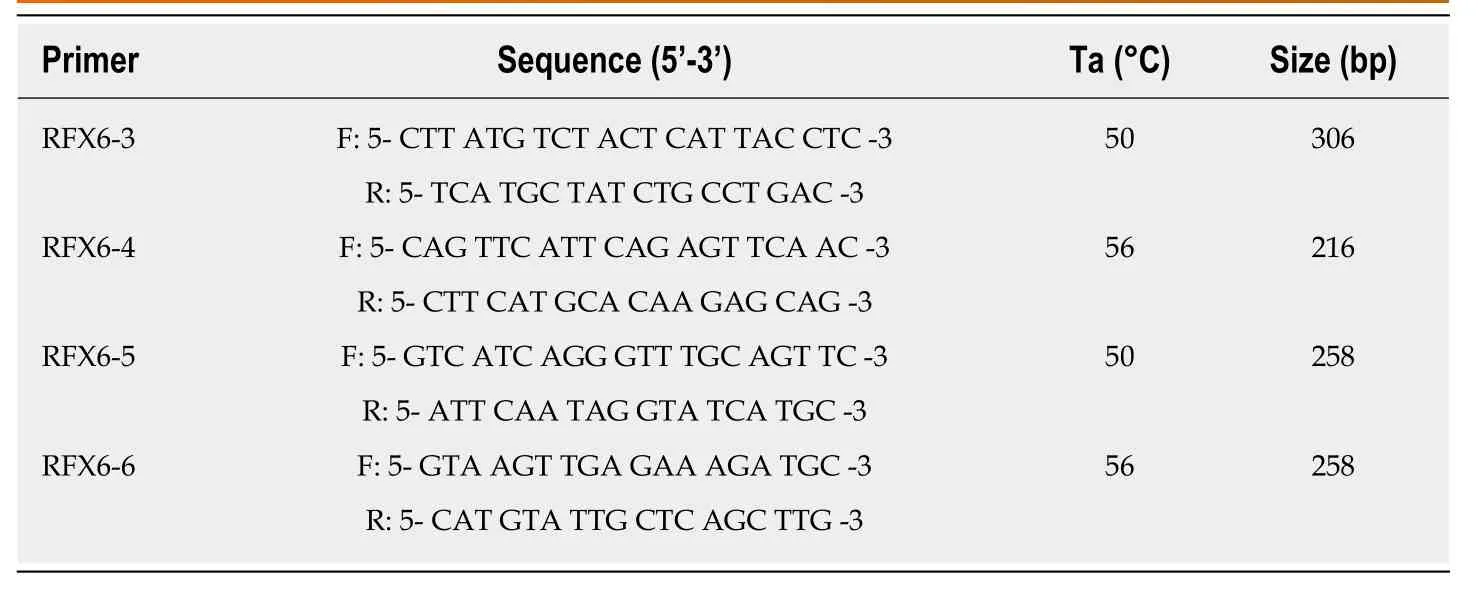
Table2 Primer sequences used in DNA amplification of DNA binding domain sequence of RFX6 gene

Table3 Association between the variant IVS6+31 C>T investigated in RFX6 gene and type 2 diabetes risk
ARTICLE HIGHLIGHTS
Research background
Diabetes mellitus is a global health challenge,which is usually associated with the loss/dysfunction of insulin-producing pancreatic beta cells.Hence,understanding the molecular mechanisms that control beta cells differentiation and function represents a major interest in the medical field.Regulatory factor X6 (RFX6) is DNA binding protein that is predominantly expressed in pancreatic islets of human and plays a key role in regulating pancreatic beta cells differentiation and insulin production,and it has been recently.RFX6 contains a highly conserved DNA binding domain which is critical for binding of RFX6 to DNA and consequently regulates the amount of messenger RNA produced by the gene.Several lines of evidence have indicated that RFX6 binding to DNA could be disrupted in diabetes.However,the mechanism by which this could happen is still unknown.
Research motivation
The presence of genetic mutations in the gene coding for the RFX6-DNA binding domain could result in inhibition of binding of RFX6 to DNA and consequently loss of function.Defining such genetic mutations will provide valuable information to diagnose,treat,prevent and cure type 2 diabetes (T2D).
Research objectives
In this study,we sought to investigate if any structural genetic mutations could be present in the RFX6-DNA binding domain in T2D patients and whether they are associated with diabetes.
Research methods
A case-control study was conducted in T2D patients and healthy volunteers.The DNA was extracted from all subjects and polymerase chain reaction (PCR) was used to amplify genomic DNA encompassing the coding sequences and intronic borders of exons 3,4,5 and 6 of the RFX6 gene,then PCR samples were analysed by DNA sequencing.
Research results
Our data showed the absence of any mutation in the exons coding for the RFX6-DNA binding domain.However,we have identified a new heterozygous single nucleotide polymorphism(IVS6+31 C>T) in the intronic region of DNA binding domain gene that is present in 9.2% and 8.5% of diabetic and control people,respectively (P= 0.97).
Research conclusions
We conclude that genetic mutations in the DNA binding domain of RFX6 are unlikely to exist in T2D.
Research perspectives
RFX6 binding to DNA is mediated by multiple of domains.Indeed,RFX6 proteins contain other conserved regions,including B,C,and D domains,which play a critical role in oligomerization of the protein and are required for DNA binding and activation.Thus,testing the other functional domains of RFX6 in future will provide more insights into the role of RFX6 in diabetes.
ACKNOWLEDGEMENTS
We would like to thank Dr Hussam Alhawari and the laboratory staff of the Molecular Biology Research Lab (MBRL) at the University of Jordan for technical support.
杂志排行
World Journal of Diabetes的其它文章
- Cataract in diabetes mellitus
- Crosstalk between gut microbiota and antidiabetic drug action
- Antidiabetic treatment on memory and spatial learning:From the pancreas to the neuron
- Targeted genotyping for the prediction of celiac disease autoimmunity development in patients with type 1 diabetes and their family members
- Burden of diabetic foot ulcer in Nigeria:Current evidence from the multicenter evaluation of diabetic foot ulcer in Nigeria
- Optimized health care for subjects with type 1 diabetes in a resource constraint society:A three-year followup study from Pakistan
