Female-biased Dispersal of the Emei Moustache Toad(Leptobrachium boringii) under Local Resource Competition
2019-03-27WenxiaWANGShichaoWEIMengjiaoCHENandHuaWU
Wenxia WANG, Shichao WEI, Mengjiao CHEN and Hua WU
Institute of Evolution and Ecology, School of Life Sciences, Central China Normal University, 152 Luoyulu, Hongshan District, Wuhan 430079, China
Abstract Dispersal is an important area of ecological and evolutionary research. Although many studies have been conducted in mammals and birds, dispersal pattern in amphibians is still unclear. To verify dispersal patterns of amphibians, an endangered species the Emei Moustache Toad (Leptobrachium boringii) was selected. In this study,we analyzed six genetic parameters: inbreeding coef fi cient (FIS), gene diversity (HS), the mean of corrected assignment index (mAIC), the variance of corrected assignment index (vAIC), relatedness (r) for all three years together and each year separately based on eight highly polymorphic microsatellite loci. Data in totaled across years and each year for 581 individuals captured during 2013-2015 revealed a significant female-biased dispersal pattern. Significantly higher FIS and HS in females, and lower mAIC and r for each year separately in females support that L.boringii displays femalebiased dispersal, although r for the total dataset and vAIC tests did not show significant differences between the sexes.Female-biased dispersal patterns may be explained by the local resource competition hypothesis.
Keywords Leptobrachium boringii, microsatellite DNA, female-biased dispersal, the local resource competition hypothesis
1. Introduction
Dispersal movements are part of the life history of animals and often involve biased patterns with one sex philopatric while the other disperses (Favre et al., 1997; Greenwood,1980). These movements typically have two forms,natal dispersal (referring to the movements from site of birth or hatching to location of the first reproduction)and breeding dispersal (referring to the movements that occur between breeding events) (Greenwood and Harvey, 1982). For populations, dispersal is a source of novelty through its influence on gene exchange and genetic diversity among populations (Bonte et al., 2012;Palo et al., 2004). Therefore, dispersal movements are important for the structure and dynamics of populations,especially for a small one (Smith and Green, 2006).
There are two different approaches to measuring sexbiased dispersal patterns: traditional methods that are based on field observations and molecular methods that rely on genetic markers. Field-based methods include radio telemetry, mark-recapture studies and passive integrated transponders that enable the movements of individuals to be monitored (Bennetts et al., 2001; Smith and Green, 2006). Genetic-based methods depend on biparental or uniparental markers (Shaw et al., 2017),which are respectively represented by inherited material(like microsatellite DNA) and by sex-specific markers(like mitochondrial DNA or sex chromosomes). If only one sex disperses, the bias can be detected by all methods mentioned above. However, the power to detect sexbiased dispersal through certain statistic approaches can be confounded if both males and females disperse. Sexbiased dispersal also will be more difficult to detect using genetic techniques when dispersal is either rare or widespread. Specifically, when the level of dispersal is too low, the genotypes of immigrants may only have a small effect on the population, or the probability that immigrants will be sampled is small. In contrast, when the level of dispersal is too high, the proportion of immigrants will be large and differences among populations will be dif fi cult to detect. Therefore, optimal tests will occur for intermediate dispersal rates (Goudet et al., 2002). One additional drawback of biparentally inherited markers is that genetic information from both sexes will be passed down to offspring after reproduction (Lampert et al., 2003) regardless of the sex of the recipient. As a consequence, some gender-specific information will be weakened (Lampert et al., 2003) unless the trends detected are supported by similar results from different methods. Sex-biased dispersal has been extensively investigated in birds and mammals (Greenwood, 1980).Although exceptions exist (Berg et al., 2009; Gour et al., 2013), birds primarily display female-biased dispersal (Lebigre et al., 2010), whereas mammals have significantly male-biased patterns (Croteau et al., 2010).Such dispersal patterns have been explained based on the relationship between the sex-biased dispersal pattern and mating system (Dobson, 1982; Greenwood, 1980;Pusey, 1987). Studies have also suggested that sexual dimorphism, parental care and other factors can also influence sex-biased dispersal (Trochet et al., 2016).Mechanisms to account for these patterns can be broadly classi fi ed into three categories: local resource competition(LRC) (Greenwood, 1980), local mate competition(LMC) (Dobson, 1982) and inbreeding avoidance (IA)(Pusey, 1987). Recently, cooperative behavior of kin as this influences local resource enhancement (LRE)has been identified as the fourth hypothesis (Lawson Handley and Perrin, 2007). Unlike birds and mammals,little is known about the extent of sex-biased dispersal in amphibians, and no general dispersal pattern has been identi fi ed. Two species of frog exhibited a female-biased(Austin et al., 2003; Palo et al., 2004), whereas studies of a salamander and a frog showed male-biased (Helfer et al., 2012; Lampert et al., 2003), one additional study found no sex-biased dispersal (Smith and Green, 2006).
The Emei moustache toad (Leptobrachium boringii)is a Chinese endemic endangered species that lives near mountain streams at high altitudes (Fei et al., 2010). It is an explosive breeder with mating and spawning that last for 2-3 weeks from mid-March to early April. Males are larger than females and grow fi ve to eight keratinized nuptial spines on one side of the upper lip, whereas females have no spines (Zhang et al., 2016). These male spines are used to fi ght with other males during territory defense or when acquiring mates. Males arrive first in spring and occupy fl at rocks in breeding areas. Females subsequently arrive to the streams and join the mating.During one breeding season, a female produces only one clutch, whereas a male can fertilize the egg masses of multiple females. Females usually lay eggs on the sites with existing egg masses, which means all the eggs in one male’ territory are in the same place. Once breeding completed, males will provide parental care until early June, whereas females leave the stream after oviposition.Based on the sex-speci fi c body size and life history traits of the Emei moustache toad, we predicted that females would be more likely to disperse to enhance their capacity to compete for resources with males. In this study, we used eight microsatellite loci to test whether L.boringii shows a female-biased dispersal pattern and to explain the mechanism forming this pattern.
2. Materials and Methods
2.1. Sampling We studied this species along a rocky stream (29.655°-29.830° N, 109.696°-110.164° E) in the Badagongshan National Nature Reserve, Hunan,China. The study stream extended approximately 500 m and elevation ranged from 1383 to 1501 m. The stream was densely populated with rocks that provided breeding and hiding places for L. boringii. At the beginning of the breeding season, we searched on a daily basis for L. boringii by gently turning rocks in the stream. When toads were captured, we clipped approximately 1 mm3of webbing from the hind foot (Grafe et al., 2011). We used single-use gloves, sterilized stainless steel scissors and antiseptic to minimize infection. After the sampling, we marked individuals by tying cotton colored lines about 0.5 mm in diameter on their waist to distinguish toads that had been sampled. All toads were then released as soon as possible. Gender was distinguished based on two morphological characteristics of L. boringii: males are usually larger than females (although sub-adult males are similar to adult females, their limbs are stronger and more muscular); males grow keratinized nuptial spines (among adults) or smaller developing spines (among sub-adults)during the breeding season. Samples were stored in 95%ethanol in 2 mL Eppendorf tubes, then stored at -20°C until use.
2.2. Laboratory procedures Whole genomic DNA was extracted using TIANamp Genomic DNA kits (TIANGEN Biotech Co. Ltd., Beijing, China) with the steps on the specification. Tissues were dried at room temperature,ground in a 1.5 mL Eppendorf tube with proteinase K digestion treatment at 56°C for 2 hours, followed by absolute ethanol precipitation and Spin Columns CB3 purification, then dissolved DNA in ddH2O. Products for each sample were resolved by PAGE (1% Agarose,TSINGKE, Beijing, China) on BIO-RAD PowerPacTMBasic and BIO-RAD Gel DocTMXR+. When DNA bands stained by ethidium bromide (EB) could be seen clearly,DNA samples were stored at a temperature of -20°C for use. Polymerase chain reactions (PCRs) were performed with eight microsatellite loci, including LSMT1, LSMT3,CHA1, CHA9, VIB-B4, VIB-C10, VIB-D5, VIB-D7 (Hu et al., 2012; Bi et al., 2010; Wang et al., 2011), and all the forward primers were 5′-labeled with FAM, TAMRA,or HEX fl uorescent dyes. PCR conditions were the same as described in relevant literature (Hu et al., 2012; Bi et al., 2010; Wang et al., 2011). The reactions were carried out in 10 μl systems containing 0.8 μl genomic DNA, 10 μmol/L (0.3 μl) of each primer (forward and reverse), 5 μl r-Taq Mix (TaKaRa Bio Inc., Otsu, Shiga, Japan) and 3.6 μl of ddH2O. PCR products were analyzed on an ABI 3730XL DNA Analyzer (Applied Biosystems, Foster,CA, USA) and scanned with GeneMapper 4.0 (Applied Biosystems, Foster, CA, USA). The results were read with GeneMarker 1.3 (SoftGenetics, State College, PA,USA). All samples were read at least three times in order to reduce arti fi cial error.
2.3. Genetic diversity analysis Data were tested for null alleles using Micro-Checker 2.2.3 with a 99 % con fi dence interval and 1 000 randomizations (Van Oosterhout et al., 2004). We used data obtained from microsatellite loci to analyze the genetic diversity. Hardy-Weinberg Equilibrium (HWE) and Linkage Disequilibrium (LD)were assessed using GENEPOP 4.2 (Austin et al., 2003).The levels of significance for multiple comparisons were Bonferroni corrected (Rice, 1989). Genetic diversity including number of alleles (A), observed heterozygosity(HO), expected heterozygosity (HE), allele richness (AR)and polymorphism information content (PIC) were calculated using Cervus 3.0 (Kalinowski et al., 2007).
2.4. Dispersal analysis We did the dispersal analysis by microsatellite data totaled across years and each year,respectively. For analysis by the total dataset across three years, we compared the sex differences of several genetic indices using FSTAT 2.9.3 with 10 000 randomizations(Goudet, 2001). The inbreeding coefficient (FIS) is a measure of genotypic frequency when the population matches HWE. A positive FISwas predicted for the dispersing sex (Goudet et al., 2002; Palo et al., 2004).Members of the immigrating sex should exhibit a higher FISthan the philopatric one. For the dispersing sex,individuals from a site will be a mixture of residents and immigrants, and should therefore exhibit higher gene diversity (HS) (Austin et al., 2003; Goudet et al., 2002;Lampert et al., 2003). The assignment index (AI) is a measurement reflecting the probability that an individual originated from the locality. Because of the differences in genetic diversity among populations, a corrected assignment index (AIC) is provided, which reflects the frequency of an individual genotype within the sampled population (Favre et al., 1997). In the case of sex-biased dispersal, the mean of corrected assignment index values(mAIC) for the dispersing sex should be lower than the philopatric sex (Lampert et al., 2003). In contrast, the variance of the corrected assignment index (vAIC) is expected to be larger for the dispersing sex because the members sampled include both residents and immigrants(Austin et al., 2003; Goudet et al., 2002; Lampert et al.,2003). Finally, we inferred the different levels of dispersal between sexes by comparing relatedness (r). The dispersing sex should exhibit lower levels of relatedness than the philopatric sex (Lampert et al., 2003). In brief,the FIS, HSand vAICshould be higher whereas mAICand r should be lower in the dispersing sex than in the philopatric sex. For analysis by the each year’s data separately, we compared r between sexes. The values of r were calculated with CONANCESTRY 1.0 using five moment and two likelihood estimators (Wang, 2011).
3. Results
3.1. Genetic diversity analysis A total of 581 individuals(311 females, 270 males) was sampled in this study.Speci fi cally, there were 57 females and 82 males in 2013,126 females and 91 males in 2014, 128 females and 97 males in 2015 (Table 1). The results showed significant null alleles at the loci for LSMT1 (Year 2013-2015: P <0.05) and VIB-D7 (Year 2013-2015: P < 0.01, Table 2).When the frequency of a null allele is greater than 0.2,the marker locus probably should be dropped from the analysis (Dakin and Avise, 2004). However, considering that only two values were slightly larger than 0.2 (bold in Table 2) in one estimator and increasing number of polymorphic loci can improve the analysis power for detecting sex-biased dispersal, we chose not to discardany loci. Therefore, eight microsatellites were genotyped to perform for further analyses. After incorporating a Bonferroni correction, no significant deviations from HWE and LD were found. The average number of alleles was 8.875 (range 3-17), the average allele richness was 7.837 (range 3-14.236), the average observed heterozygosity and expected heterozygosity were 0.686 and 0.718, respectively (Table 3).

Table 1 The sample information of the Emei moustache toad (L.boringii) in the Badagongshan National Nature Reserve.
3.2. Dispersal analysis To test a sex-biased dispersal pattern in L. boringii, we analyzed and compared six genetic parameters based on microsatellite data. When we examined the total dataset from three years, three of the fi ve tests supported our hypothesis that L.boringii displays female-biased dispersal (Table 4). Both FISand HSwere significantly different between sexes, all values were higher for females than males (FIS: female F = 0.069,male F = -0.008, P = 0.001; HS: female F = 0.716, male F = 0.703, P = 0.015). Conversely, mAICwas significantly higher in males than in females (mAIC: female F = -0.318,male F = 0.366, P = 0.012). The vAICand r showed no

2013EMZC Oosterhout 0.053 0.096 0.002 0.031 0.019 0.013 0.059 0.174 Chakraborty 0.058 0.088 0.001 0.022 0.022 0.005 0.049 0.213 Brook field 1 0.047 0.074 0.001 0.021 0.015 0.004 0.046 0.156 Brook field 2 0.047 0 0 0.02 0.045 0.004 0 0.185 Null Present yes no no no no no no yes 2014EMZC Oosterhout 0.046 0.043 0.001 0.017 0.058 0.011 0.025 0.106 Chakraborty 0.05 0.043 0.005 0.022 0.054 0.007 0.024 0.121 Brook field 1 0.041 0.031 0.004 0.019 0.036 0.005 0.022 0.097 Brook field 2 0.041 0.086 0.072 0.059 0.036 0 0 0.118 Null Present yes no no no no no no yes 2015EMZC Oosterhout 0.019 0.045 0.003 0.013 0.013 0.016 0.035 0.165 Chakraborty 0.019 0.035 0.002 0.002 0.013 0.013 0.032 0.201 Brook field 1 0.016 0.029 0.001 0.002 0.009 0.01 0.029 0.153 Brook field 2 0.016 0 0 0.023 0 0.01 0 0.17 Null Present no no no no no no no yes
Note: Bold character means the significant test results and the values greater than 0.2.significant difference (vAIC: female F = 10.230, male F = 8.777, P = 0.360; r: female F = -0.0004, male F =0.004, P = 0.264). When we examined each year’s data separately, two out of seven r showed significantly higher(P < 0.05) values in males than in females (Table 5).
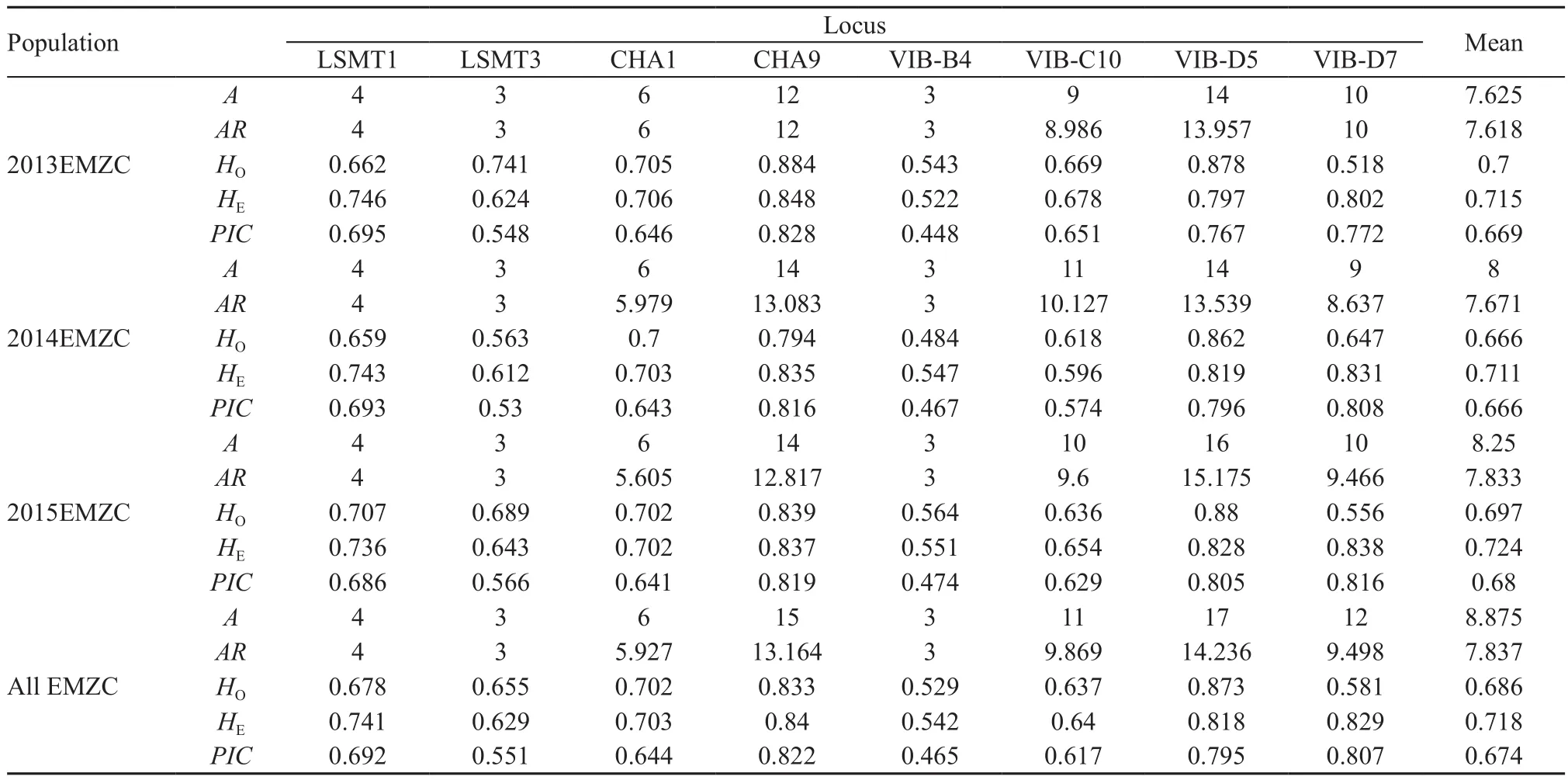
Table 3 The results of gene diversity based on eight microsatellite loci in the Emei moustache toad (L. boringii).

Table 4 Test results by the total dataset for sex-biased dispersal in the Emei moustache toad (L. boringii).
4. Discussion
4.1. Genetic diversity We found that the genetic diversity based on microsatellite data in L. boringii was higher or similar than other amphibians. For example, the HO(0.484-0.884) and HE(0.522-0.848) of L. boringii were higher than the moor frog (Rana arvalis) (HO= 0.34, HE= 0.38) (Vos et al., 2001), whereas these parameters are similar to some sex-biased dispersal species such as the tungara frog (Physalaemus pustulosus) (HO: 0.359-0.95,HE= 0.617-0.929) (Lampert et al., 2003). In addition, the mean values of the polymorphism information content were greater than 0.5. These results indicate that L.boringii was highly genetically diverse.
4.2. Dispersal For analysis by the total dataset, although r and vAICvalues did not show significant differences between sexes, we found that several parameters (FIS,HSand mAIC) provided support for our hypothesis that the Emei moustache toad exhibits a female-biased dispersal pattern. The higher FISand HSin females than in males strongly suggested that females exchanged more genetic material among populations than males, and that females are more likely to be immigrants. The lower mAICin females means that females were less likely to originate from the locality than males. For analysis by the each year’s data, greater r in males indicated that the relatedness in male-male pairs was higher than in femalefemale pairs within each population. In conclusion, both the data across all years and for each year separately suggested that L.boringii displays a female-biased dispersal pattern. According to Goudet et al. (2002),the power of different indices varies with dispersal rate,bias intensity, number of loci and samples. As vAICis particularly sensitive to rare alleles, when the intensity of dispersal is lower than 10%, the power of vAICto indicate the presence of dispersers would be stronger than other statistics. Conversely, r is more sensitive when immigrants constitute a larger proportion of the population. In addition, mAICis somewhere between vAICand r, and it will retain enough power when the intensity of dispersal drops to 80: 20 (immigrants: residents).Moreover, the sex-speci fi c information will be weakened when analyses are based on microsatellite DNA, which could be the reason why vAICand some r values did not show significant differences between sexes. Other studies have shown similar results. For instance, values of vAICbetween sexes were not significant whereas other tests indicated female-biased dispersal in the bullfrog (Rana catesbeiana) and the common frog (Rana temporaria) (Austin et al., 2003; Palo et al., 2004). In the study of the tungara frog (P. pustulosus), only the vAICrevealed a significant difference whereas other parameters(FST, FIS, mAIC) showed non-significant differences between males and females (Lampert et al., 2003).In addition, similar dispersal patterns have also been observed in the common frog (R. temporaria) (Palo et al.,2004) and the Columbia spotted frog (Rana luteiventris)(Pilliod et al., 2002). In contrast to L. boringii, the tungara frog (Lampert et al., 2003) and the alpine salamander(Salamandra atra) (Helfer et al., 2012) exhibit a malebiased dispersal.
According to game theory, individuals tend to dispersal when the benefits outweigh the costs (Bonte et al., 2012).Several ultimate reasons for female-biased dispersal in L. boringii may be speculated. First, the local resource competition (LRC) hypothesis predicts that individuals show more philopatric when they benefit more from their original site and the immigrants disperse to avoid kin competition for resource (Greenwood, 1980).From previous studies, sex-biased dispersal patterns have some relationship to territorial resource possession and parental care. The gender which monopolized resources will be less likely to spread because of more benefits (Greenwood, 1980; Lawson Handley and Perrin,2007; Trochet et al., 2016). Through field observations,we noted that males of the Emei moustache toad occupy territories to get beneficial resources, thus, they are more inclined to stay. In L.boringii, males who arrive at oviposition sites early will occupy high quality habitats to attract females. Besides, males stayed beside the nests after females left to take care of offspring and get other mating opportunities. By doing this, they not only protected the eggs against predators, but also produced more offspring in the same place by multiple mating.
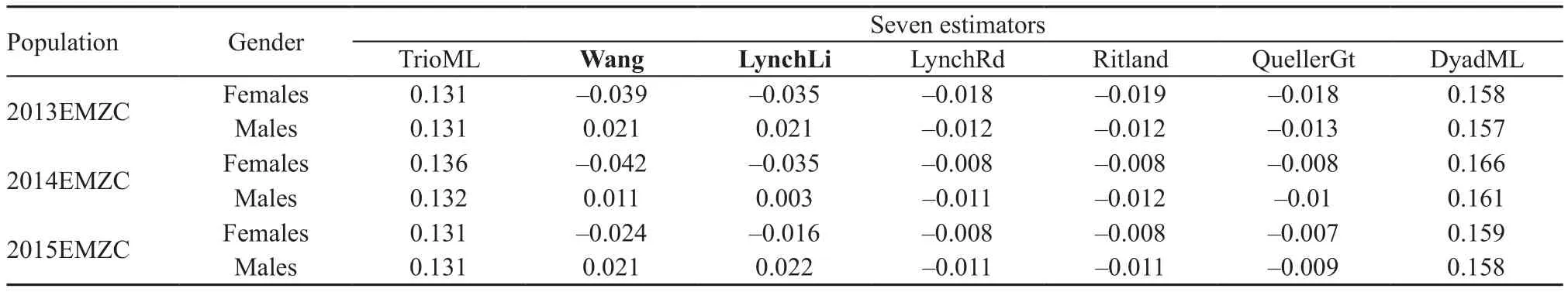
Table 5 The relatedness of females and males in each year separately.
Therefore, males invest heavily in offspring and have significant advantages (more food and mates, less risk and energy expenditure) when they continue to live in a familiar environment. At this time, staying at home sites may be detrimental to females as appropriate flat rocks were limited in breeding areas and males occupied these areas in advance. Moreover, males were stronger than females so that males have certain advantages when obtain food. Females need to look for new high quality habitats and more food. Therefore, we concluded that the female-biased dispersal pattern in L.boringii is more in line with the local resource competition hypothesis.Second, the local mate competition hypothesis (LMC)indicates that individuals disperse to obtain more mates and avoid competition with related individuals (Dobson,1982). The local resource competition (LRC) can be classified together with the local mate competition(LMC) as kin selection. The difference is that the LMC emphasizes the impact of local mate defense on sexbiased dispersal and is applicable to mammal dispersal in polygynous and promiscuous species, such as the male-biased dispersal of California ground squirrels(Spermophilus beecheyi) (Dobson, 1982; Lawson Handley and Perrin, 2007). Based on the mating system of the Emei moustache toad, males can mate multiple times with different females. It means that females have plenty of opportunity for mating. Therefore, this hypothesis is not completely consistent with the femalebiased dispersal in Emei moustache toad. Third, kin cooperation including local resource enhancement (LRE)may help individuals to acquire or defend mates and territory in resource defense systems, and thereby promote philopatry. This hypothesis seems to unfavorable to dispersal under the benefits of kin cooperation. However,it may bring particular benefits to sub-adults and weak members when individuals disperse as a group. Although most animals tend to dispersal individually, disperse as a group is know in some species. In white-faced capuchins(Cebus capucinus) for example, 82% of males leave their natal site with relatives (Jack and Fedigan, 2004).
The same situation occurs in lions, males disperse in the company of related individuals (Pusey and Packer, 1987).Kin cooperation plays a crucial role in social species that have a stronger capability of kin-recognition, such as mammals (Lawson Handley and Perrin, 2007). Therefore,this hypothesis can probably be excluded in amphibians due to the lack of sociality. For the fourth hypothesis,the inbreeding avoidance hypothesis (IA) explained that inbred offspring have a higher frequency of harmful alleles than that of non-inbred individuals. Therefore,individuals disperse to avoid mating with related individuals (Pusey, 1987). This is common in birds and mammals. From previous studies, inbreeding avoidance has negligible influence on the sex-biased dispersal patterns in a population (Perrin and Mazalov, 2000)because that dispersal should not be biased only to avoid inbreeding (Smith and Green, 2006). This result is similar to studies previously, the sex responsible for defending territory and parental care is more likely to stay (Dobson,1982; Greenwood, 1980). For example, the bullfrog (R.catesbeiana) exhibits female-biased dispersal, with which the males are responsible for defending territory (Austin et al., 2003). In contrast, the wood frogs (Rana sylvatica)without territoriality or parental care behavior exhibit an unbiased dispersal pattern (Berven and Grudzien, 1990).In summary, the Emei moustache toad is femalebiased dispersal and the ultimate reasons may be the local resource competition. As dispersal has a vital effect on gender structure, genetic structure and population dynamics, a thoroughly understanding of dispersal behaviors will help us understand more about population structure for endangered species.
Acknowledgements This work was fi nancially supported by the National Natural Science Foundation of China(No. 31770405). All samples were collected with the permission of the Management Bureau of Badagongshan National Nature Reserve. The experiments were performed under an animal ethics approval granted by the Central China Normal University.
杂志排行
Asian Herpetological Research的其它文章
- Resurrection of the Genus Leptomantis, with Description of a New Genus to the Family Rhacophoridae (Amphibia: Anura)
- A New Species of the Genus Trimeresurus from Southwest China(Squamata: Viperidae)
- Macroecological Patterns of Climatic Niche Breadth Variation in Lacertid Lizards
- Microhabitat Segregation of Parapatric Frogs in the Qinling Mountains
- Embryonic Growth and Yolk Depletion during Incubation in the Chinese Skink, Plestiodon chinensis
- Investigating the Effectiveness of Road-related Mitigation Measures under Semi-controlled Conditions: A Case Study on Asian Amphibians
