Cladophora mats in a Crimean hypersaline lake: structure,dynamics, and inhabiting animals
2018-12-22AlexandrPRAZUKINElenaANUFRIIEVANickolaiSHADRIN
Alexandr V. PRAZUKIN, Elena V. ANUFRIIEVA, Nickolai V. SHADRIN
The A. O. Kovalevsky Institute of Marine Biological Research of RAS, 2 Nakhimov Av., Sevastopol 299011, Russia
Abstract Filamentous green algae play an important functional role in element cycling and productivity in the different water bodies. In hypersaline lakes and lagoons of the Crimea, filamentous green algae are present and form bottom and floating mats that occupy large areas with high biomass, up to 4–5 kg(wet biomass)/m 2. Cladophora spp. dominated in those mats. Five species of filamentous green algae(Chlorophyta) in Lake Chersonesskoye: Cladophora vadorum (Aresch.) Kütz., C. siwaschensis C. Meyer,C. echinus (Biasol.) Kütz., Ulothrix implexa (Kütz.) Kütz., Rhizoclonium tortuosum (Dillw.) Kütz., and seagrass (Angiospermae) Ruppia cirrhosa (Petagna) Grande were found. Cladophora spp. and R. cirrhosa were found in the lake throughout the year, other types of algae were encountered episodically. In most cases the biomass of bottom mat exceeded that of the floating mat. In general, the total biomass of the bottom and floating mats in the lake areas at depths up to 30 cm was in a stable range of values from 100 to 290 mg(dry weight)/cm 2. Animal and infusorian average abundance in mats reach high values: infusorians—up to 15 000 000 ind./m 2, Cletocamptus retrogressus (Copepoda, Harpacticoida)—up to 730 000 ind./m 2,Eucypris mareotica (Ostracoda)—up to 91 000 ind./m 2 and Chironomidae larvae (Insecta, Diptera)—up to 140 ind./m 2. Those values were much higher than in the plankton.
Keyword: green algae mats; hypersaline lake; photosynthesis; invertebrates
1 INTRODUCTION
Filamentous green algae play an important functional role in element cycling and productivity in different water bodies, reaching high biomass. Due to eutrophication during the last decades they have become more abundant in lagoons (Curiel et al.,2004), lakes (Higgins et al., 2012), estuaries (Gubelit and Berezina, 2010), streams (Okada and Watanabe,2002), and reservoirs (Dondajewska et al., 2007),creating some problems for the human population.Cladophoraspp. are among most frequent of those green algae. Phosphorus is generally the limiting nutrient for filamentous green algae development in lakes (Higgins et al., 2012; Song et al., 2017). Only few reports have mentioned nitrogen-limited filamentous green algae growth in aquatic systems(Song et al., 2017); heterotrophic nitrogen-fixing bacteria amongCladophoraepibionts may be one of the factors enabling their development (Young et al.,2010; Zulkifly et al., 2012).Cladophoracan forms different types of mats, which are very complicated systems including different epibiontic organisms(bacteria, microalgae, infusorians) and free living protists and animals (Pavlovskay et al., 2009; Zulkifly et al., 2012; Shadrin and Anufriieva, 2013). To avoid phosphorus limitation,Cladophoracan accelerate P regeneration by excreting alkaline exophosphatase(Song et al., 2017). Epibiontic microalgae and bacteria as well as mat-dwelling infusorians and animals also accelerate phosphorus cycling, thus promoting high development of filamentous green algae.

Fig.1 Lake Chersonesskoye in the Crimea (a–c) and schemes of algal mat biomass distribution in the lake (d, e)
In hypersaline lakes and lagoons worldwide,filamentous green algae are present andCladophoraspp. dominate when lake salinity exceeds 100 g/L(Gordon et al., 1980; Hammer et al., 1983; Velasco et al., 2006). In hypersaline lakes and lagoons of the Crimea, they may form bottom and floating mats and occupy the large areas with high biomass, up to 4–5 kg(wet biomass)/m2(Ivanova et al., 1994; Prazukin et al.,2008; Balushkina et al., 2009; Shadrin and Anufriieva,2013). Animal and infusorian abundance may reach high values in mats (Pavlovskay et al., 2009; Shadrin et al., 2016, 2017).Cladophoramats also influence the aquatic environment, modifying evaporation rate,regimes of temperature, oxygen, pH, Eh, etc. (Prazukin et al., 2008; Shadrin, 2017). Despite this, our knowledge on these mats is very limited. The main goal of this article is to describe and discuss the results of our multiyear study on the structure and dynamics ofCladophoramats in a small Crimean hypersaline lake.
2 MATERIAL AND METHOD
2.1 Study area
In 2003–2017, complex studies were conducted on Lake Chersonesskoye (44°35′09″N, 33°23′39″E) in the southwestern part of the Crimea, the largest peninsula in the Black Sea (Fig.1); their main results were published (Kolesnikova et al., 2008; Prazukin et al., 2008; Senicheva et al., 2008; Batogova et al.,2009; Pavlovskay et al., 2009; Gubanov and Bobko,2012; Prazukin, 2009, 2015), but many results related to mats have not been published before. The lake is shallow, has an oval-oblong shape, the area of the water is 0.05 km2with a catchment area of 0.92 km2;the average depth is 0.38 m and the largest—about 1.3 m. In summer, due the water level decrease, a shallow small part of the lake is separated by an isthmus. High spatial-temporal variability of abiotic parameters was observed. Intensive water heating is observed starting in April and reaches its maximum in August (30–43°C). In winter the temperature can drop below 0°C; the lowest value in the coldest winter was little below -7°C. The maximum salinity value during the observation period was 360 g/L in the‘small lake’ and 160 g/L in the other lake part; the minimum salinity was 35 g/L. The ratio of major ions in the water, as in other hypersaline lakes of marine origin, does not differ from that in the Black Sea. The average daytime pH value in the lake was 8.64; the maximum value can reach 10.0, which is due to the high intensity of photosynthesis. In 2005, the absolute minimum pH (7.73) in upper layer of mat was registered in September; it was associated with the development of sulfate reduction processes under floating mats. In 2005, annual average daytime absolute concentration of oxygen was 5.21 mL/L(102% of saturation). In August 2005, in the presence of hydrogen sulfide in a deeper layer, an absolute maximum of the relative oxygen content (202%) was recorded. In 2005, average phosphate concentration(PO4) was 0.58±0.3 μmol/L, nitrate concentration(NO3) was 2.35±2.06 μmol/L and silicate concentration(SiO3) was 7.94±7.74 μmol/L.
The lake surface was almost completely covered by the floatingCladophoramat at the observed point.During the period of observations on the lake phytoplankton, 61 microalgae species were recorded:Dinoflagellata: 19 species, Bacillariophyceae: 15,Chlorophyta: 9, Cyanobacteria: 7, Chrysophyta: 6,Cryptophyta: 3, Euglenoidea: 2. Seventy species of benthic diatoms were noted. Macrophytes were represented by 6 species, 5 of them belong to the filamentous Chlorophyta and one species—to seagrasses (Angiospermae). Twenty-four infusorian and more than 40 animal (Nematoda, Turbellaria,Rotifera, Diptera, Coleoptera, Crustacea) species inhabit the lake. As it was shown before, most part of benthic animals in hypersaline waters (Chironomidae,Harpacticoida, Ostracoda, Nematoda) transits to planktonic life due to high density of water, hypoxic/anoxic condition near bottom, salt sedimentation(Shadrin and Anufriieva, 2013; Shadrin et al., 2017).This lake is not exclusion from such regularity.
2.2 Sampling and sample processing
Observation and sampling of mats, inhabiting them animals and zooplankton was conducted in 2000–2017 in 2–4 points of the lake. Only in 2005, sampling and observations were made in the summer-autumn period on five transects several times, in 5–10 points on every transect (Fig.1). On transects, starting from the shore and up to depths of 50 to 55 cm, samples were taken with a cylindrical sampler with a crosssectional area of 452 cm2, which allows seaweed to be taken horizontally throughout the water column. The algae biomass samples were washed in freshwater,dried with filter paper and weighed on an electronic balance WT-250 (Techniprot, Poland). To determine the dry mass, the algae were dried at a temperature of 105°C to constant weight and weighed on the same balance. On the basis of the obtained data, the volumetric concentration of plant mass in different parts of the lake was calculated from the equations(Prazukin, 2015):

whereCW: dry weight algal biomass per unit of mat volume, mg (dry weight)/cm3;Wd: dry mass of mat sample, mg;V: mat sample volume, cm3.

wherem1: wet weight algal biomass per unit of water surface in sampling point, kg (wet weight)/m2;Ww:wet weight of mat sample, kg;S0: the surface area from which the sample was taken, m2.

wherem2: dry weight algal biomass per unit of mat surface, mg (dry weight)/cm2.
Animals were selected from the clumps of algae of known mass. The relative number of animals in the mats was determined by dividing the number of counted individuals by the mat sample weight.Zooplankton samples were collected by filtration of 100–150 L of water through a plankton net with a mesh size of 110 μm. Samples were fixed with 4%buffered formalin solution and examined under an Olympus SZ-ST stereomicroscope. Identifications were made using a Carl Zeiss Axio Scope A1 light microscope. Totally more than 150 plankton and mat samples with animals were analyzed. In the lake,benthos, infusorians and plankton were studied in 2005–2006; methods and partly results were described previously (Kolesnikova et al., 2008; Pavlovskay et al., 2009; Anufriieva and Shadrin, 2012). The authors use these results here. At the sampling points,temperature and salinity were evaluated. Temperature was measured by a PHH-830 electronic thermometer,salinity—by a manual Kelilong WZ212 refractometer.The water temperature was measured directly in the floating and bottom mats and in the water outside the mat, the salinity in the floating mats, and the incident radiation at a distance of 3 cm above the surface of the floating mat. Data were subjected to standard statistical processing in Grapher-7, Excel 2007.

Fig.2 Cladophora mats in different seasons of the year in Lake Chersonesskoye
3 RESULT
3.1 Structure and dynamics of the mats in the lake
In 2000–2017, the authors found five species of filamentous green algae (Chlorophyta) in the lake:Cladophoravadorum(Aresch.) Kütz.,C.siwaschensisC. Meyer,C.echinus(Biasol.) Kütz.,Ulothrix implexa(Kütz.) Kütz.,Rhizocloniumtortuosum(Dillw.) Kütz, and seagrass (Angiospermae)Ruppiacirrhosa(Petagna) Grande. Usually each sample contained between one and three species; their composition varied in time.Cladophoraspp. andR.cirrhosawere found in the lake throughout the year, other types of algae—during some periods.
Every year, excluding only three of 2000–2017,Cladophoraspp. formed bottom and floating mats at salinities at least up to 200 g/L, accompanied by the purple bacteriaChromatiumandEctothiorhodospira(identification was made by L. Gerasimenko, see Shadrin et al., 2008) during certain periods. For July and August 2005, maps were made for the distribution of mats in the lake water area (Fig.1d, e). In the lake sections whereRuppiawas present,RuppiaandCladophorajointly formed a bottom mat. According mat biomass and structure, characteristic areas in the lake were identified (Table 1). In July and August 2005,Cladophoramats occupied almost the entire water area of the lake (Fig.2d). The coastal area of the lake up to depths not exceeding 20 cm (the 1st lake area) covered 13% of the total lake area in July and had high biomass (4.1–5.5 kg (wet weight)/m2,Fig.2c) contributing 29% of total mat biomass in the lake. In August, coastal areas (lake areas 7 and 8)occupied 9% of the lake area and gave 19% of the total macroalgae biomass. In both surveys, in the central part of the lake, mat biomass varied between 1.1 and 2.8 kg (wet weight)/m2). In July, 69% of the lake area was covered by floating mats, and in August—only 37%. In July and August, the total stock of macrophytes was close—30.98 and 31.13 tons (wet weight), respectively; 82%–84% of total biomass was in the bottom mats. In the winter months there was only a thin strip ofCladophoraspp. along the shore (Fig.2a); and small thickets ofR.cirrhosain some sections. In the middle of March,Cladophorabottom and floating mats were beginning to form along the coastline (Fig.2b), and by mid-August these can occupy 60%–70% of the lake area (Fig.2d). As a rule,Cladophoraspp. dominated in the lake macrophyte biomass in most part of the water area,and only in certain parts, thereRuppiawas present in equal proportion. For 17 years of our observations a complete lack ofCladophoraspp. in the winter season in three times; and only three times, we observed a very low abundance ofCladophorain the following summer-autumn period without mat forming.
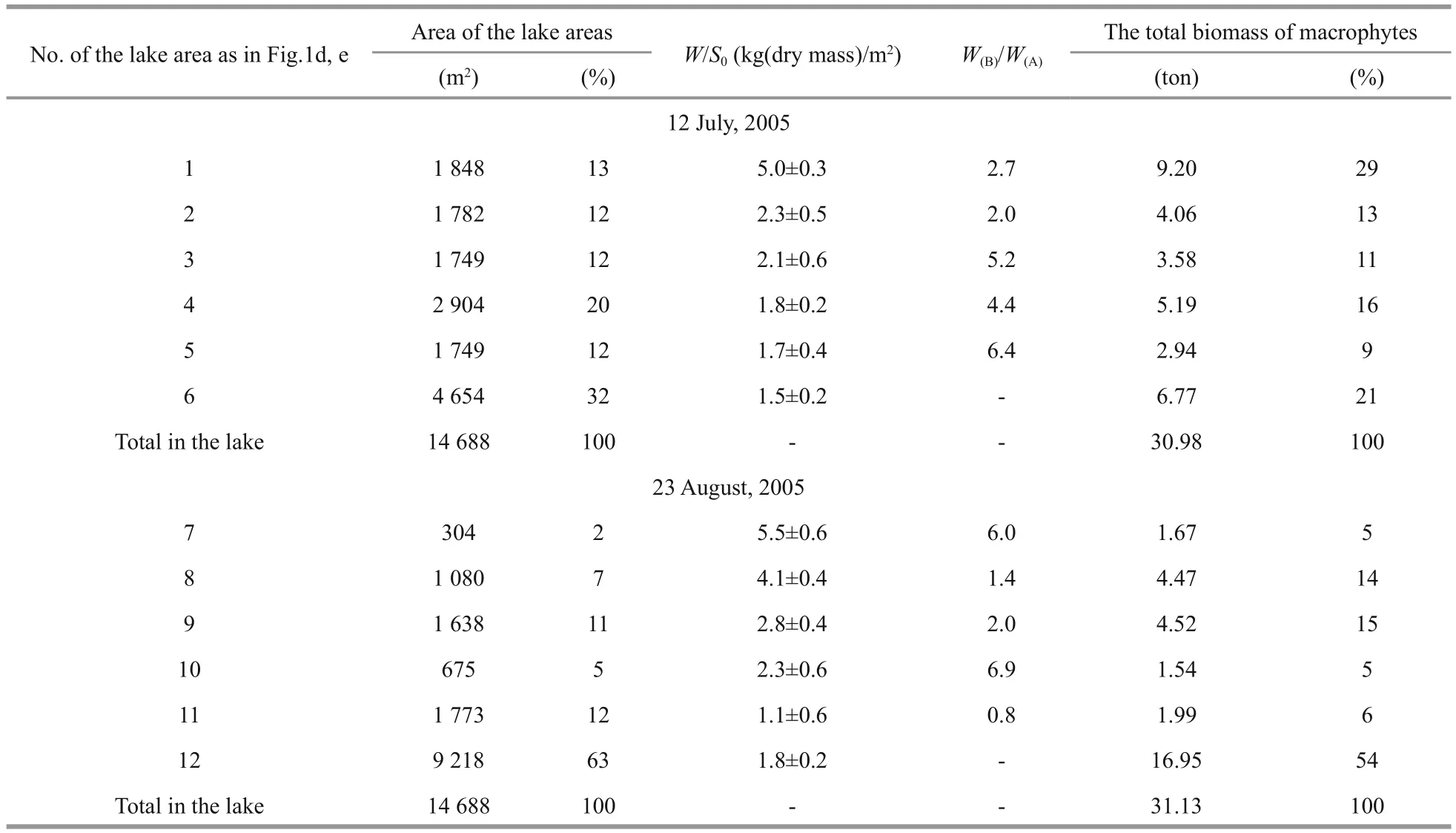
Table 1 Characteristics of Cladophora mats in different parts of the Lake Chersonesskoye
3.2 Vertical structure of the mats
Distribution ofCladophorawas not homogeneous;it is possible to distinguish bottom and floating mats(Fig.3a). The ratio between them varied with distance from the shore and depth (Fig.4, I–V). In turn, each mat had a characteristic vertical structure. Near the lake coastline to depths not exceeding 10 cm, a dense multi-layered mat was formed (Fig.4; ’A’, I (a), I (b),Fig.3a–d). As a rule, there was a dense (36–67 mg(dry)/cm3) interlacing ofCladophorafilaments and shoots ofRuppia(Fig.5). In the vertical structure of the mat, two or three layers were detected, differing in density and color. The top layer (A1, Fig.4) was either a dense crust of algae, covered with a thin layer of mineral salts (Fig.3b), or a thin, loose mass of algae with white or light-green color (Fig.3d, e). Below there was a relatively thick layer of dark-green algae(A2, Fig.4), sometimes with a large number of different-sized caverns of unknown origin (Fig.5,Fig.3d–f) often there were also channels in A1–A2 made by animals (Amphipoda, Coleoptera). The third, lower layer (A3, Fig.4) was a layer of decaying macrophytes. Visually here the cladophora filaments were green, black and purple-pink in color (Fig.3b, c).In the general mass, the purple-pink color predominated, which was due to the presence of anoxygenic phototrophs—purple bacteria.

Fig.3 Floating and bottom mats near the coast in Lake Chersonesskoye in August
On the lake sections with depths of 10–20 cm,there were the floating mat (A) and the bottom mat(B) (Fig.4, II). The morphological structure of the floating mat was identical to the structure of the mat located near the coastline (Fig.4, I (a), I (b)).Ruppiamay ‘pierce’ floating mats with its shoots, making it motionless (Fig.5). The bottom mat covered the space from the lower boundary of the floating mat to the bottom and, in turn, was divided into the upper (B1)and lower (B2) layers (Figs.4, 5). The top layer was formed by the freely floatingCladophora‘balls’ of green or salad-green color (Fig.3g). In the lower layer,there were algae with signs of decay, colored green,black and purple-pink.
At sites with a depth of more than 20–25 cm, the relative location of the floating and bottom mats and their characteristic vertical structure were variable. In some cases, both mats were simultaneously present (Fig.4, (III (a), III (b)), and there was a space between them, free from macrophytes (Fig.4, zone ‘C’). In other cases, only the bottom (Fig.4, IV) or only the floating (Fig.4, V) mat formed. The vertical structure of the bottom mat (Fig.4, IV) was similar in many respects to the structure of the floating mat: a thin surface layer of light green color (B1 (a)), beneath which there is a layer of freely floatingCladophoragreen ‘balls’ (B1 (b)) and below that—a layer of decaying algae (B2). In all cases, below the zone ‘B’there was a ‘liquid’ layer of bottom sediments of biogenic origin (Fig.5; G1)—a mineral-organic complex formed as a result of the decomposition of algae and animal remains that emerged over a short period of time, for example, for the spring-summer season. A thick ‘solid’ layer was below this (Fig.5;G2), accumulated during the period of existence of the lake.

Fig.4 Different states (I–V) of the vertical structure of the floating (A) and bottom (B) Cladophora mats at different depths( Z) in the lake and different off shore distances ( L)
Figure 6 shows the structural characteristics of mats, located at different distances from the shore with different depth. The mat thickness (h(A))decreased with increasing depth (Fig.6a). The thickness of the mat lying near the coastline and the floating mat near the shore reached 6–7 cm; the floating mat of the open lake sections did not exceed one centimeter. The concentration of dry mass per volume unit of the mat (CW) also changed with depth(Fig.6b). In September, in the shallow lake part, to the depth not exceeding 30 cm, two maximum ofCWwere detected: the first near the shore, and the second at a considerable distance from the shore. In the latter case, the floating mat was a thin (0.9 cm), dense(85 mg (dry)/cm3) continuous algae film at the waterair boundary. In other cases (Fig.6b), especially in the open part, the floating mat was not dense and had an open-work appearance, while the volumetric concentration of dry matter in floating mat varied from 4.6 to 30 mg (dry)/cm3. The amount of dry mass calculated per unit surface (W(A)/S0,W(B)/S0) varied widely and decreased with depth (Fig.6c, d). In most cases, the bottom mat biomass exceeded that of the floating mat (Fig.6f). In general, the total biomass of the bottom and floating mats in the lake areas at depth up to 30 cm was within a stable range of values from 100 to 290 mg (dry)/cm2(Fig.6e).
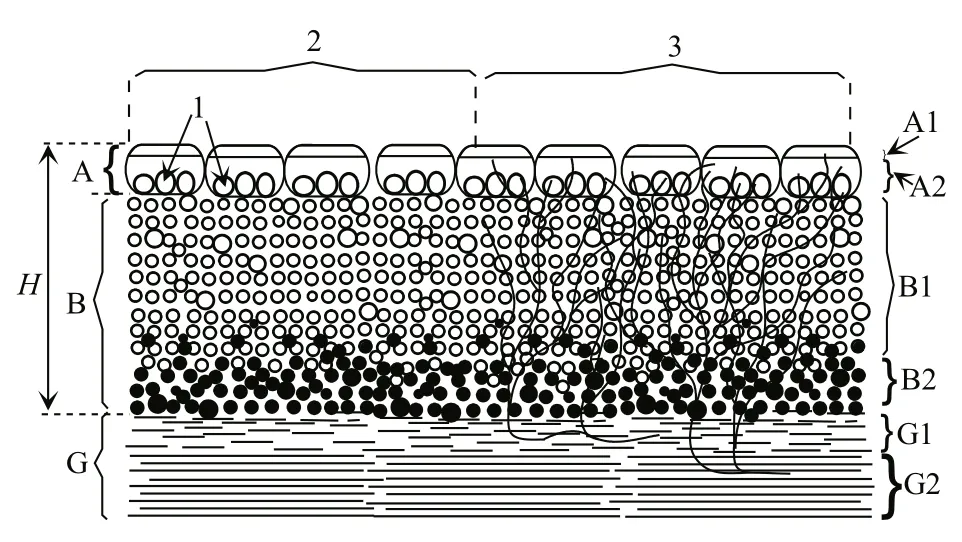
Fig.5 The vertical structure of the floating and bottom mats at depths 10–20 cm
3.3 Animals in the Cladophora mats
All animal species found in the lake were encountered also in the mats. Some groups were found only in the mats such as Amphipoda (Orchestia gammarellus(Pallas, 1766),O.mediterranea(Costa,1853)) and several species of Coleoptera. The most common and abundant in mats were the same animal species as in the plankton: HarpacticoidaCletocamptus retrogressusSchmankevitsch, 1875 and OstracodaEucyprismareotica(Fischer, 1855). Amphipoda and some Coleoptera made the channels in dense mats near the lake coastline, promoting ventilation of the mats. In all cases, abundance of animals in the mats was higher by order of magnitude than in the plankton far from mats (Table 2). Under mats, animal abundance was much low or animal were absent due to H2S presence.
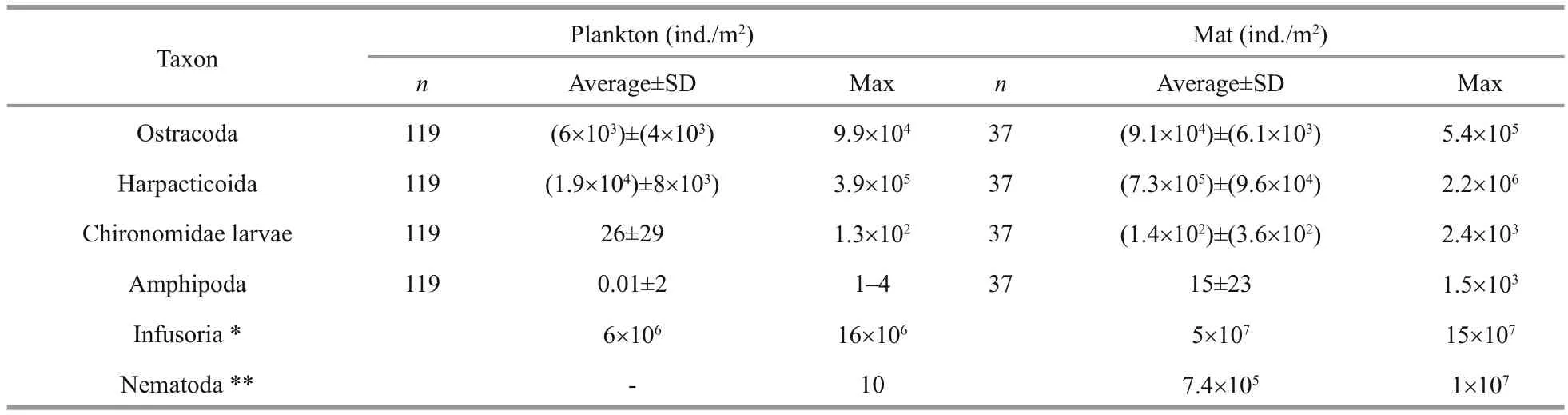
Table 2 Animal and infusorian abundance in the plankton and the mats of Lake Chersonesskoye in 2004–2017
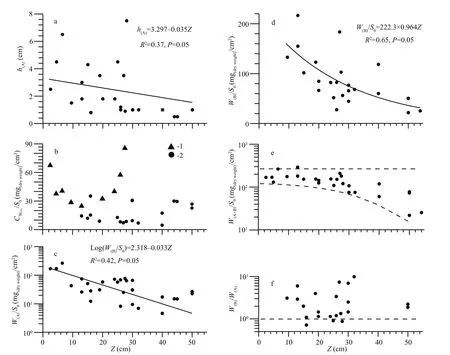
Fig.6 The structural characteristics of Cladophora mats at different depths in the lake ( Z) varying with off shore distance( L) (see also Fig.4)
4 DISCUSSION
The formation of mats of filamentous algae usually in shallow water bodies with high concentrations of nutrients as in our case and their effects on the environment have been discussed (McGlathery et al.,1997; Scheffer et al., 2003; Prazukin et al., 2008;Prazukin, 2015). There are competitive relationships for solar radiation and nutrient elements between the bottom and floating mats (McGlathery et al., 1997;Scheffer et al., 2003; Green and Fong, 2015). In our case, the strongest competitive relations between the floating and bottom mats were shown to exist in the shallow part of the lake (Fig.4).
Such competition can be explained by an observed decrease of intensity of light, temperature, dissolved oxygen, C/P and C/N ratios with the depth (Phillips et al., 1978; O’Neal and Lembi, 1983; Eiseltová and Pokorný, 1994; Gubanov and Bobko, 2012; Saunders et al., 2012). In a floatingPithophoramat, only 1% of the incident light reached a depth of 1 cm (O’Neal and Lembi, 1983), in someCladophoramats, the percentage of sun radiation penetrating was 2%(Eiseltová and Pokorný, 1994), and in the dense mats ofChaetomorphalinum(Müller) Kütz., light penetration was restricted to 8 cm (Krause-Jensen et al., 1996). Due to the high levels of solar radiation the upper layers of the mats may suffer from oxidative stress and photoinhibition (Jiang and Qiu, 2005),whereas algae in the lower layer may have to cope with very low levels of photosynthetically active radiation (Vergara et al., 1997). Earlier, the authors showed that the concentration of carotenoids and chlorophyllaandbper biomass unit in the layer of cladophora ‘balls’ (B1) was approximately twice as high as in the lower layer of the floating mat (A1)(Prazukin et al., 2008), which indicates an adaptive response of algae to a reduced light intensity in the lower part of the mat (Berner et al., 1989). It was shown that the intensity of photosynthesis in a mat decreased from 0.7 μg C/(mg·h) (upper 1–4 cm) to 0.2 μg C/(mg·h) at a depth of 9 cm (Prazukin, 2009).Perhaps below the layer ofCladophora‘balls’ (B1),oxygenic photosynthesis was practically impossible.In the ‘B2’ layer, decomposition processes predominantly occurred, theCladophorafilaments were mostly colored black and purple-pink due to the presence of anoxygenic phototrophs—purple bacteria(Fig.3b, c, e). That’s why highest O2concentration(200% of saturation) was observed in an upper layer of a mat, and H2S was detected under the floating mat(Gubanov and Bobko, 2012), and anoxic zones are a common phenomenon under such mats in the Crimean hypersaline lakes (Pavlovskay et al., 2009; Shadrin and Anufriieva, 2013; Shadrin et al., 2016).
Animals are a common component of the mat,reaching abundance as high as in mats of other Crimean hypersaline lakes (Ivanova et al., 1994;Balushkina et al., 2009; Shadrin and Anufriieva,2013; Shadrin et al., 2016, 2017). These animals are highly halotolerant and can dwell at salinities up to 280–350 g/L (Anufriieva, 2014, 2015; Shadrin et al.,2017). Such a high concentration of infusorians and animals (Table 2) and their small sizes (high intensity of metabolism) provide a high rate of nutrient regeneration in mats. Inhospitable conditions on the bottom (oxygen deficit first) push benthic animals transit to live in mats or plankton.Cladophoramats contribute to the high number and diversity of animals in them first providing food—a lot of epibiontic microalgae on cladophora filaments, etc.
Ecosystems of some hypersaline lakes in the Crimea, like the lake studied, can be in various alternative states, when in one of them, the main primary producer isCladophoramats, and in the other—phytoplankton (Ivanova et al., 1994;Balushkina et al., 2009; Shadrin and Anufriieva,2013; Shadrin, 2014; Prazukin, 2015). Ecosystems with mats are characterized by an increased intensity of primary production. For example, in Lake Tobechikskoye in August 2005, there was intensive development ofCladophoramats, and their primary production (47 g C/(m2·d)) exceeded the primary production of plankton by 69 times (Balushkina et al.,2009). A similar thing was noted for Lake Bakalskoye(Shadrin and Anufriieva, 2013). It should also be noted that the intensive development ofCladophoraspp. with mat formation in the lakes contributes to the growth of the number and diversity of animals and to the decrease of the sedimentation and resuspension of bottom sediments, thereby increasing the transparency of the water (Balushkina et al., 2009; Shadrin and Anufriieva, 2013).
Floating and bottom mats constitute a whole integrated spatially organized system, which in addition toCladophoraspp. includes different groups of organisms: plants, algae, animals, and bacteria.Our results allow us to conclude that in the vertical structure of the mat (Fig.2), there is a zone where photosynthesis predominates (A, B1) and a decomposition zone (B2); together these two processes (production and decomposition of organic matter) within the boundaries of the mat form a cycle of matter, which determines the structural and functional integrity of the system. Heterotrophic organisms (numerous bacteria, infusorians and animals) play an important role in the regeneration of nutrients within the mat. Internal circulation of substances takes place inside the mat contributing to the maintenance of high algal biomass. Due to all the above, the aquatic ecosystem with floating mats is a stable system (Scheffer et al., 2003) capable to exist in a wide range of temperatures and salinities. Further comprehensive study of these unique communities will expand our understanding of the patterns of functioning, dynamics and transformation of aquatic ecosystems.
6 ACKNOWLEDGMENT
The author thanks to anonymous reviewers for valuable comments. The study was carried under support of the Russian Academy of Sciences for the A. O. Kovalevsky Institute of Marine Biological Research of RAS.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Neuroanatomy and morphological diversity of brain cells from adult crayfish Cherax quadricarinatus*
- On the influence of season and salinity on the phenology of invertebrates in Australian saline lakes, with special reference to those of the Paroo in the semiarid inland
- Man-made plutonium radioisotopes in the salt lakes of the Crimean peninsula
- Antimony speciation at the sediment-water interface of the Poyang Lake: response to seasonal variation*
- Seasonal variations of phosphorus species in the Tuohe River,China. Part I. Sediments*
- The characteristic pattern of multiple colored layers in coastal stratified lakes in the process of separation from the White Sea*
