Effects of seawater acidi fication on the early development of sea urchin Glyptocidaris crenularis*
2018-08-02ZHANYaoyao湛垚垚HUWanbin胡婉彬DUANLizhu段立柱LIUMinbo刘敏博ZHANGWeijie张伟杰CHANGYaqing常亚青LiCong李骢
ZHAN Yaoyao (湛垚垚) , HU Wanbin (胡婉彬) , DUAN Lizhu (段立柱) , LIU Minbo (刘敏博) ,ZHANG Weijie (张伟杰) , CHANG Yaqing (常亚青) , , Li Cong (李骢)
1 Key Laboratory of Mariculture & Stock Enhancement in North China’s Sea, Ministry of Agriculture, Dalian Ocean University,Dalian 116023, China
2 College of Basic Medical Science, Dalian Medical University, Dalian 116044, China
Abstract In this study, we evaluated the effects of CO 2-induced seawater acidi fication on fertilization,embryogenesis and early larval development in the sea urchin Glyptocidaris crenularis, that inhabits subtidal coastal areas in northern China. The range in seawater pH used in experiments was based on the projections of the Intergovernmental Panel on Climate Change (IPCC), to the year 2100. A natural seawater treatment (pH nbs=7.98±0.03) and three laboratory-controlled acidi fied treatments (OA 1, ΔpH nbs=-0.3 units;OA 2, ΔpH nbs=-0.4 units; OA 3, ΔpH nbs=-0.5 units) were used in experiments. Results show that: (1) there was a negative effect of seawater acidi fication on fertilization and on the percentage of abnormal fertilized eggs; (2) the size of early cleavage stage embryos decreased in a dose-dependent manner with decreasing pH; (3) both the hatching rate of blastulae and the survival rate of four-armed pluteus larvae decreased as pH declined; (4) larval abnormalities including asymmetrical development, changes in the length of skeletal elements, and corroded spicules were observed in all seawater acidi fied-treatments compared with the control. These data indicate that seawater acidi fication has a negative impact on the early development of G. crenularis, and supports the hypothesis that the response of echinoderms to ocean acidi fication (OA)varies among species. Further research is required to clarify the speci fic cellular mechanisms involved.
Keyword: seawater acidi fication; Glyptocidaris crenularis; early development; calci fication
1 INTRODUCTION
Over the past century, sea urchins have been widely used as model organisms in studies on developmental biology, evolutionary biology and physiology(Lawrence, 2013). In addition, sea urchins play a critical ecological role in marine communities as grazers and as prey (Pearse, 2006).
Ocean acidi fication (OA), caused by excessive carbon dioxide (CO2) emissions, has become a topic of concern worldwide (Dupont and Pörtner, 2013).Sea urchins are a good subject for ocean acidi fication studies due to their world-wide distribution and their carbonate structures, which are considered vulnerable and sensitive to climate stressors (Dupont et al.,2010). Recent research efforts evaluating the effects of CO2-induced seawater acidi fication on sea urchins have mainly focused on fertilization kinetics (Dupont and Thorndyke, 2013; Hardy and Byrne, 2014),pluteus development (Dupont and Thorndyke, 2013;Chan et al., 2015), skeleton biomineralization(Brennand et al., 2010; Byrne et al., 2013b), gene expression pattern alteration (Todgham and Hofmann,2009; Martin et al., 2011; Hammond and Hofmann,2012), and the response of the acid-base regulatory system (Stumpp et al., 2012; Calosi et al., 2013). To date, about 2.7% of sea urchin species have been studied with respect to the impact of CO2-induced seawater acidi fication on different life-history stages;including gametes, embryos, larvae, juveniles and adults (Dupont and Thorndyke, 2013). Recent laboratory and field studies indicate that some sea urchins are more robust to seawater acidi fication than others, even within a population of the same species(Dupont and Thorndyke, 2013). This indicates that there are inter-speci fic and intra-speci fic variability in the responses of sea urchins to CO2-induced seawater acidi fication.
The sea urchin Glyptocidaris crenularis, which is the only species of the Glyptocidaris genus, is distributed throughout the northern Yellow Sea and the Sea of Japan (Chang et al., 2004). To date, there is no information available on the response of G. crenularis to seawater acidi fication. Therefore, to provide useful information describing how CO2-driven seawater acidi fication affects early developmental processes in the sea urchin G. crenularis, a lab-based experiment was conducted.Four experimental treatments, which were based on the Intergovernmental Panel on Climate Change(IPCC) projection forecasting a decrease in ocean pH of about 0.3–0.5 units by 2100 (IPCC, 2013), were setup. The experimental treatments included one representing current natural oceanic conditions with a pHnbsvalue of 7.98±0.03 (control) and three nearfuture OA-scenarios with pHnbsvalues of 7.68±0.03(OA1), 7.58±0.03 (OA2) and 7.48±0.03 (OA3). Rates of fertilization, successful early embryonic development, the percentage of hatched blastulae and the survival rate of four-armed pluteus larvae were calculated for each of the experimental treatments.Basic spicule elements of four-armed larvae, larval morphology and the ultra-structure of calci fied spicules in larvae exposed to the acidi fied seawater treatments were examined using microscopy.
2 MATERIAL AND METHOD
2.1 Sea urchin collection and maintenance
Glyptocidaris crenularis specimens (average test diameter=33.13±1.74 mm (Mean±S.D.)) were collected from a depth of 10–50 m off Zhangzi Island in Dalian (39°3′N, 124°47′E, Yellow Sea, China). The sea urchins were transported to the Key Laboratory of Mariculture and Stock Enhancement in the North China Sea, Ministry of Agriculture at the Dalian Ocean University, Dalian, China in March 2014. The sea urchins were held in circulating seawater tanks(~1 000 L) at ambient temperature (10.85±0.49°C)and fed with kelp ( Laminaria japonica) for five days prior to the start of the experiments.
2.2 CO 2 treatments and sea water chemistry
Based on the projected ocean pH for 2100 reported by the IPCC (2013), experimental treatments representing the current pH of seawater (control) and the pH of three projected OA pH levels (OA1ΔpHnbs=-0.3 units; OA2ΔpHnbs=-0.4 units; OA3ΔpHnbs=-0.5 units) were set up. Sea urchin embryos were reared in each of the four pH treatments, using flow-through filtered seawater (FSW, 0.22 μm) with three replicate containers of embryos per treatment.Air was bubbled into each rearing container to maintain the level of dissolved oxygen (DO) at >90%.Seawater in the control treatment was filtered, but pH was not manipulated (pHnbs7.98±0.03). The pH of each experimental treatment was adjusted by injecting CO2(99.999%, Dalian Special Gases Company) with a CO2-driven seawater acidi fication system as described by Brennand et al. (2010). The seawater in each experimental treatment was stabilized as follows:OA1(pHnbs=7.68±0.03, ΔpHnbs=-0.3 units), OA2(pHnbs=7.58±0.03, ΔpHnbs=-0.4 units), OA3(pHnbs=7.48±0.03, ΔpHnbs=-0.5 units). The temperature was held at 10.85±0.49°C in all four experimental treatments. Seawater in each experimental treatment was monitored daily using a pH meter (HI9124,HANNA). Temperature, DO and salinity of each experimental treatment was determined using a water quality monitor (6920, YSI). Total alkalinity (TA) of the seawater in each experimental treatment was determined using pH titration as described in the methods provided by the State Oceanic Administration People’s Republic of China (SOA) (AQSIQ, 2007).Partial pressure of carbon dioxide ( p CO2), calcite saturation (ΩCa) and aragonite saturation (ΩAr) values for each experimental treatment were calculated based on salinity (Sal), temperature ( T), pHnbsand TA data using SWCO2software with the CO2equilibrium constants of Mehrbach et al. (1973) as re fitted by Dickson and Millero (1987). The chemical parameters of the seawater in each experimental treatment before and after the experiment are reported in the Supplementary Table S1 (statistical analysis in Table S2).
2.3 Fertilization
After exposure to air for 1 h, G. crenularis were induced to spawn using a coelomic injection of KCl(0.5 mol/L, 1–2 mL per individual), following standard methods (Chang et al., 2012). Gametes from three males and three females were collected to avoid a single male-female cross and to produce a homogeneous batch. Three experiments were conducted to improve precision ( n=3). Eggs were collected in filtered seawater (FSW, 0.22 μm) and sperm were collected dry and kept on ice in culture dishes before use, following the method of Liu et al.(2005). Prior to gamete mixing, the quality of each gamete source was checked using a microscope. Eggs were checked for shape and appearance and sperm were checked for motility. The total number of eggs and the density of sperm for each experiment was measured using a hemocytometer. In each fertilization experiment, eggs were divided into four beakers(1 L), each containing seawater at a different pH(control pH=7.98±0.03; OA1ΔpHnbs=-0.3 units; OA2ΔpHnbs=-0.4 units; OA3ΔpHnbs=-0.5 units), and each with a concentration of 50 eggs/mL. The sperm concentration was 5×104sperm/mL. In procedural controls, these gamete concentrations resulted in at least 85% fertilization success and high rates of normal development (≥75%) (Chang et al., 2004).
The eggs were pre-incubated in FSW for 15 min before the sperm was added. After 15 min, the eggs were rinsed three times in FSW to remove excess sperm and re-suspended in each of the four pH treatments. Samples were collected from each pH treatment 30 min after fertilization. Fertilization success was determined by the presence of a fertilization envelope or cleavage.
2.4 Early embryonic development
To study the effect of CO2-induced seawater acidi fication on the early embryonic development of G. crenularis; the morphology of fertilized eggs, the number of abnormal embryos (percentage), and the mean diameter of embryos at different early developmental stages (1-, 2-, 4-, and 8-cell stages)were determined. The mean diameter of embryos was calculated by measuring the diameter of the entire cell, including the fertilization membrane.Experiments were replicated three times ( n=3). For each experiment, 100 G. crenularis embryos were randomly collected from each experimental treatment after 30, 120, 210, and 240 min post-fertilization. The morphology of 300 fertilized eggs from each experimental treatment at 30 min post-fertilization was observed under a microscope (50i, Nikon, Japan).The percent of abnormal fertilized eggs were calculated using the following formula:
Percent of abnormal fertilized eggs (%)=(number of abnormal fertilized eggs 30 min post-fertilization/total number of fertilized eggs 30 min postfertilization)×100.
For each experiment, the mean diameter of 50 embryos at 1-cell, 2-cell, 4-cell, and 8-cell stages were measured using DN-2 software (Dalian Zhonghe Company).
To calculate the hatching rate of blastulae, 1 mL of the culture water in which the embryos were incubated(initial density of 50 eggs/mL) was randomly sampled after 21 h. The number of free swimming blastulae were recorded using a plankton counting frame(1 mL) under a microscope (50i, Nikon, Japan), as described by Chang et al. (2004). The formula used to calculate the hatching rate of blastulae was:
Percent of hatched blastulae (%)=(number of free swimming blastulae at 21 h/ initial density of eggs)×100.
2.5 Larval development
To examine the impact of CO2-driven seawater acidi fication on larval development in G. crenularis,we investigated larval survival, morphology, the length of basic spicule elements and larval spicule ultra-structure. Three replicated experiments were conducted ( n=3). Studies have shown that sea urchin embryos can survive up to 8 days after fertilization without suffering from malnutrition (Todgham and Hofmann, 2009; Brennand et al., 2010; Moulin et al.,2011), so we did not feed the larvae during incubation and selected 96 h after fertilization as the endpoint to calculate larval survival rates. This avoids the potential confounding in fluence induced by exogenous food (i.e. algae). To calculate larval survival rate,1 mL of the culture water that had been incubated for 96 h after fertilization was randomly sampled from beakers with an initial density of 50 eggs/mL. The number of free swimming blastulae with normal stomachs were recorded using a plankton counting frame (1 mL) under a microscope (50i, Nikon, Japan).The following formula was used to calculate the larval survival rate at 96 h post-fertilization:
Percent of larval survival (%)=(Larval density at 96 h /initial density of eggs)×100.
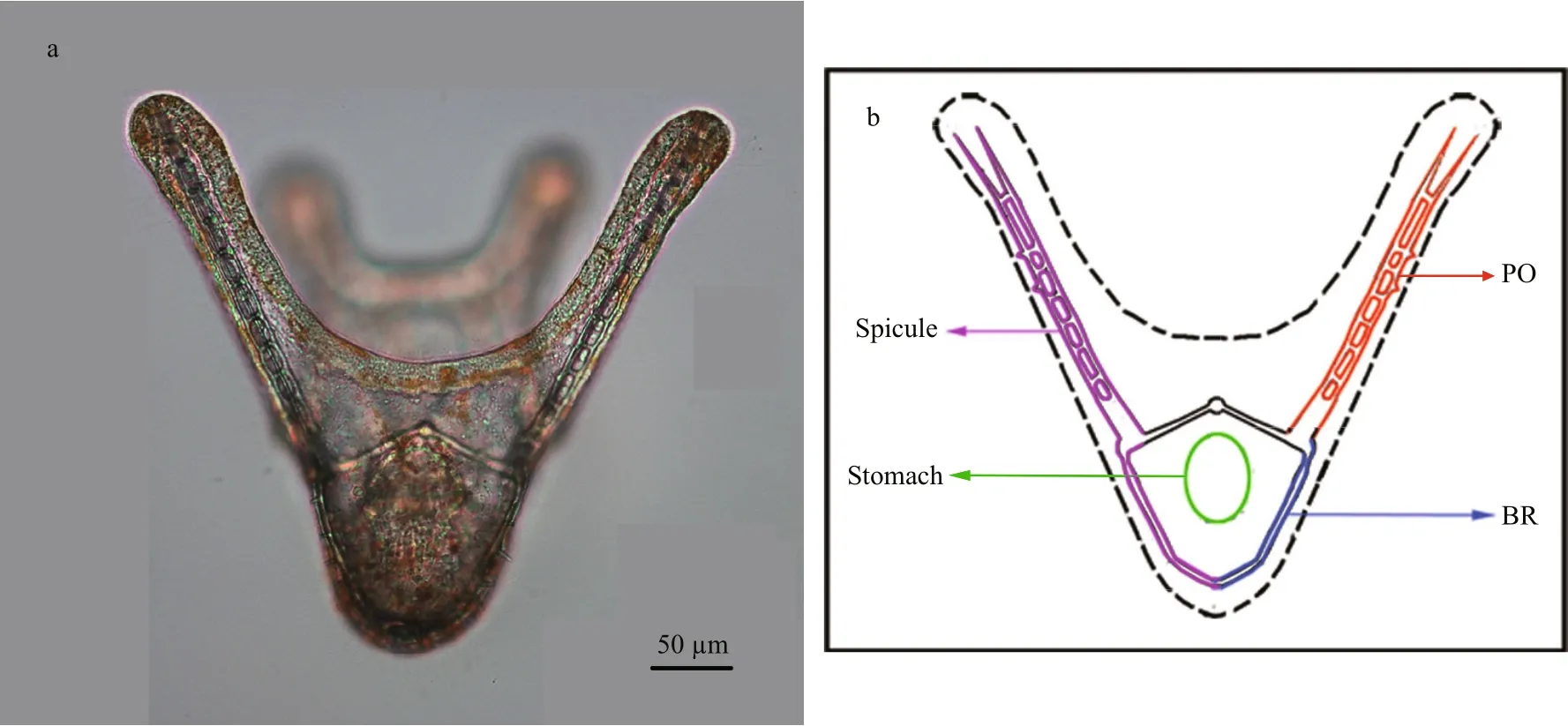
Fig.1 Four-armed larva and schematic figure of four-armed larva of G. crenularis
As four-armed pluteus larvae had well developed calci fied arms (spicules) at 96 h post-fertilization(Fig.1), basic spicule elements, such as the body rod(BR) and post oral (PO) arm, were measured. In each experiment, 50 larvae were randomly sampled from each treatment, and were fixed using the method described by Mann et al. (2010) and photographed under the microscope (50i, Nikon, Japan). The mean length of BRs, PO arms, and the distance between skeletal rods (SD) for each pluteus larva, were measured using DN-2 software (Dalian Zhonghe Company), to indicate larval development rate and calci fication. Larval symmetry was examined (as shown in Supplementary Fig.S1), and the degree of asymmetry (Ad) was calculated using the following formula:

where OLL=overall length of the longer arm, and OLS=overall length of the shorter arm.
To observe the ultra-structure of the calci fied endoskeleton (spicules), spicules of four-armed larvae were isolated as described by Mann et al. (2010). The surface sections of 100 larval spicules from specimens in each experimental group were observed using a scanning electron microscope (Quanta200, FEI).
2.6 Data analysis
All data are expressed as mean values±standard deviation (mean±S.D.). Statistical differences among measures of fertilization success, percent of abnormal fertilized eggs, mean diameter of early cleavage stage embryos, percent of hatched blastulae, larval survival rate, the degree of larval asymmetry, and differences in pluteus spicule elements (LPO, LBR, SD) among the control and all seawater acidi fied treatments were investigated using a one-way ANOVA (pH as factor,n=3) using SPSS software (version 16.0). Before analysis, Shapiro-Wilk and Levene’s tests were used to indicate normality and homogeneity of variances.All data were normally distributed and had homogenous variances. Post-hoc analyses to test differences among signi ficant means were conducted using a Tukey test. The level of signi ficance was P <0.05.
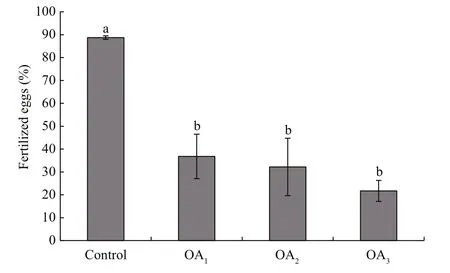
Fig.2 The impact of seawater acidi fication on fertilization success in G. crenularis
3 RESULT
3.1 The impact of seawater acidi fication on fertilization and early embryonic development in G. crenularis
At 30 min post-fertilization, the percentages of fertilization success in experimental treatments were 88.72%±0.79% (control), 36.79%±9.69% (OA1treatment), 32.17%±12.56% (OA2treatment), and 21.70%±4.60% (OA3treatment) (Fig.2). Statistical analyses revealed a dose-dependent decrease in fertilization success with decline in pH, and signi ficantdifferences in fertilization success were observed between the control and all acidi fied-seawater treatments (Table 1). Abnormal fertilized eggs with a rough vitelline membrane and non-uniform egg yolk were observed in all experimental treatments. The percentage of abnormal fertilized eggs ranged from 7.69%±4.38% in the control to 36.85%±1.61% in the OA3treatment, and showed an increasing trend as seawater pH decreased (Fig.3, Table 2). The diameter of embryos sampled at different embryo-cleavage stages (1-, 2-, 4-, and 8-cell stage) showed a decline in size as pH decreased (Fig.4, Table 3).
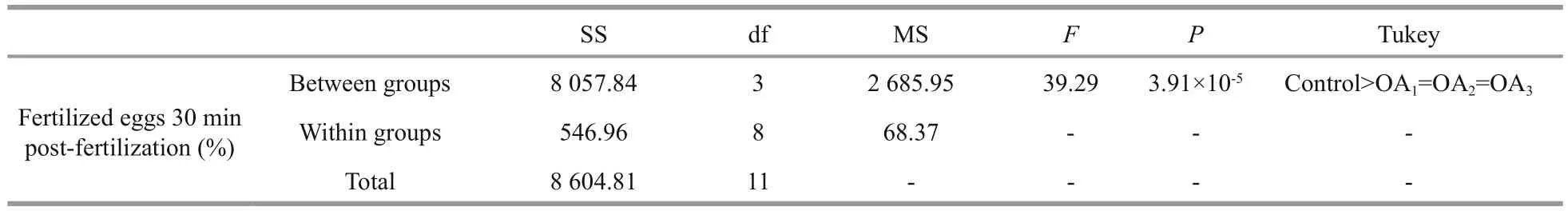
Table 1 One-way ANOVA results for the impact of seawater acidi fication on fertilization success in G. crenularis
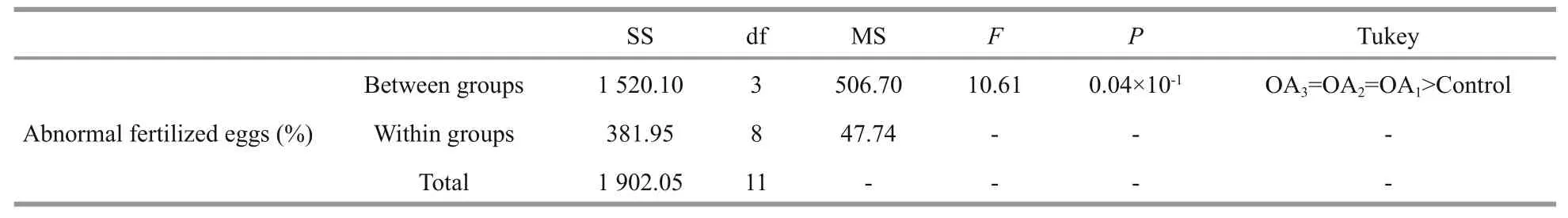
Table 2 One-way ANOVA results for the impact of seawater acidi fication on the percent of abnormal fertilized eggs in G. crenularis
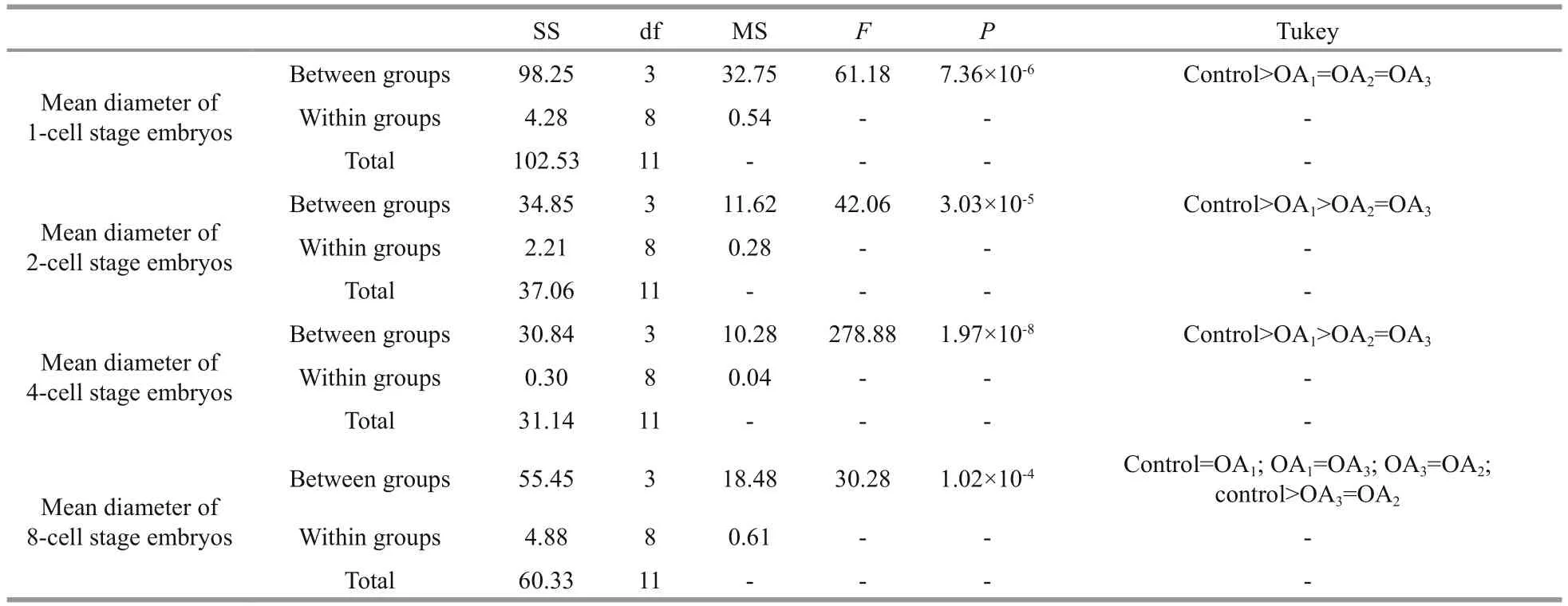
Table 3 One-way ANOVA results for the impact of seawater acidi fication on the mean diameter of embryos at different early cleavage stages in G. crenularis
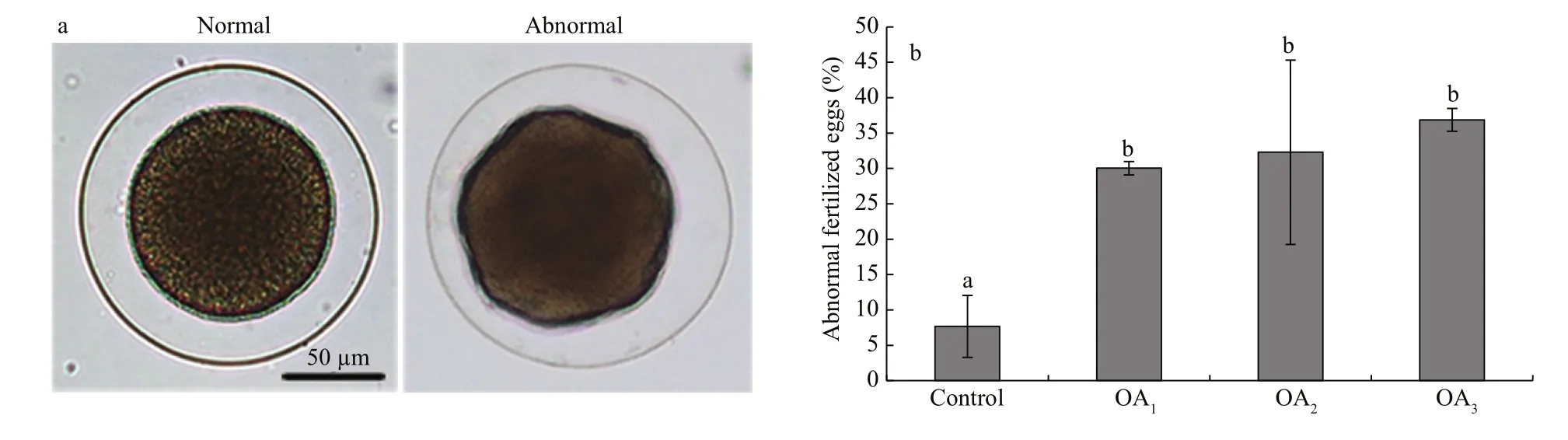
Fig.3 The impact of seawater acidi fication on fertilized eggs in G. crenularis
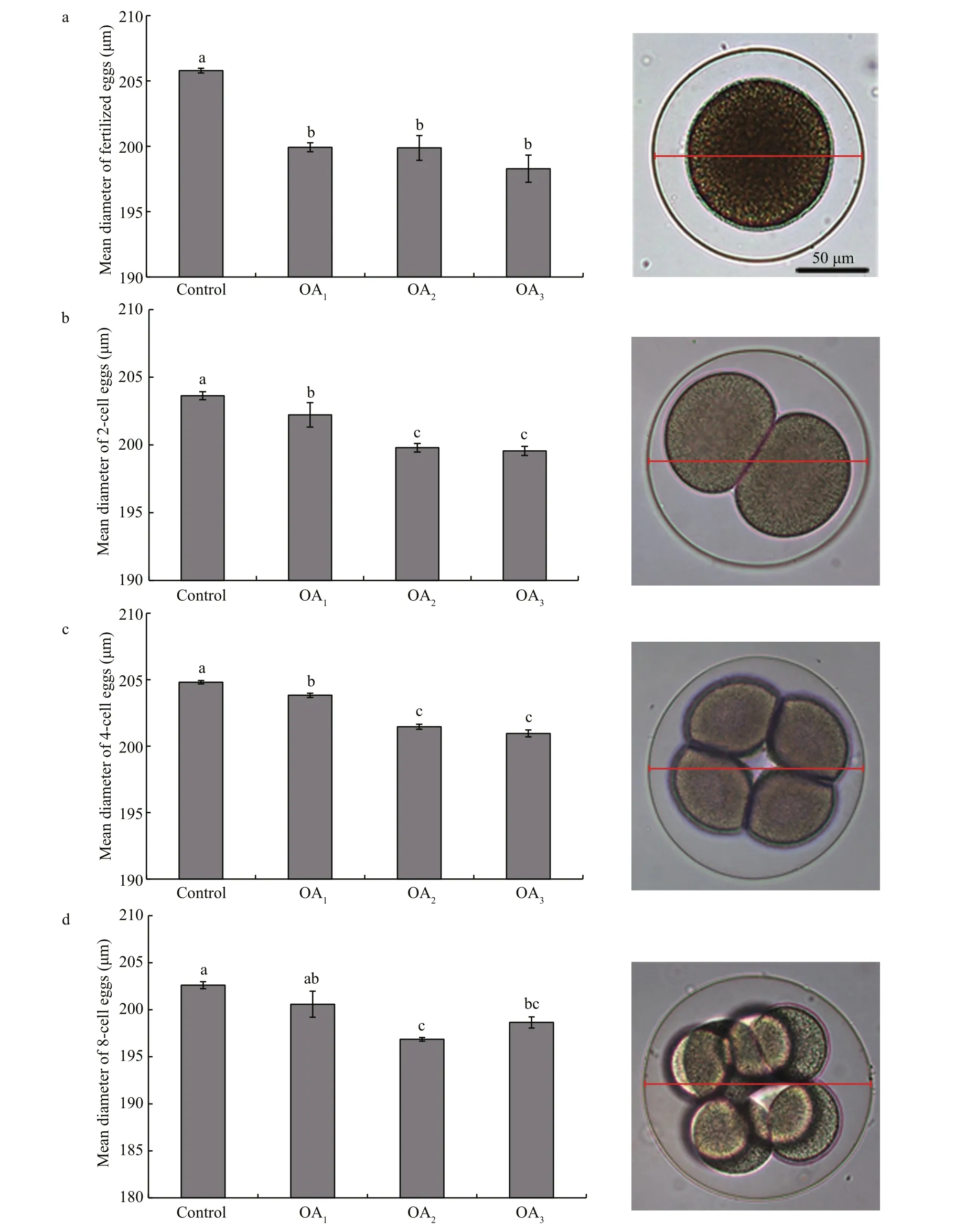
Fig.4 The impact of seawater acidi fication on the mean diameter of embryos at different early cleavage stages in G. crenularis
3.2 The impact of seawater acidi fication on the hatching rate of blastulae and on the larval survival rate of G. crenularis
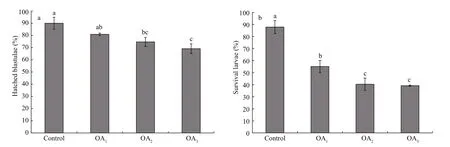
Fig.5 The impact of seawater acidi fication on the percentage of hatched blastulae and the survival rate of four-armed larvae in G. crenularis
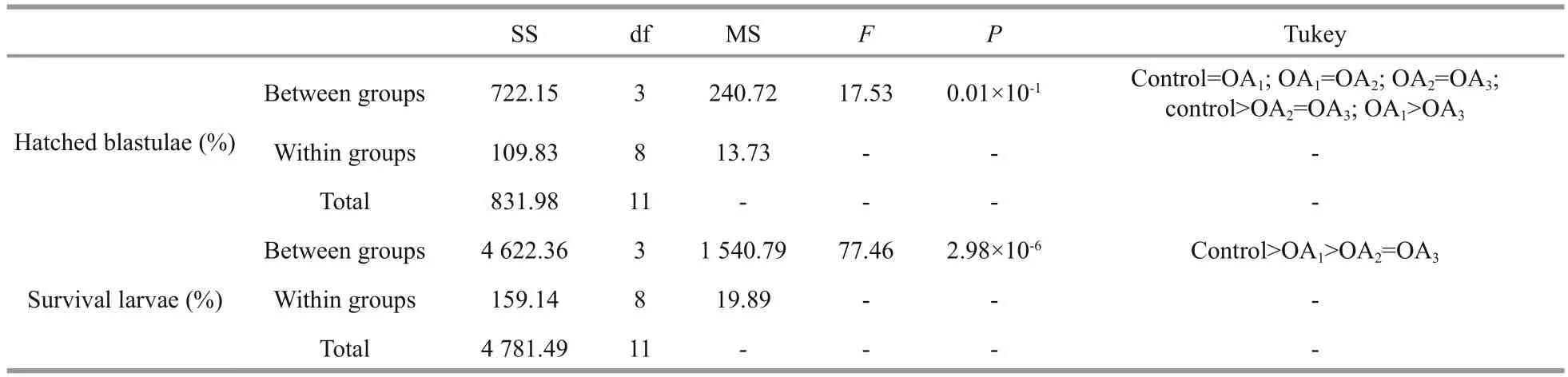
Table 4 One-way ANOVA results for the impact of seawater acidi fication on the percentage of hatched blastulae and the survival rate of four-armed larvae in G. crenularis
The impact of seawater acidi fication on late embryonic and early larval development was determined by calculating the survival rates of hatched blastulae and of four-armed pluteus larvae. A dosedependent decline in the survival of both the percent of hatched blastulae and the percent of four-armed larvae was observed in the acidi fied treatments, compared with the control (Fig.5, Table 4). The hatching rate of G. crenularis blastulae decreased as pH became more acidic and statistical differences were observed between OA2and OA3compared with the control (Fig.5a, Table 4). The survival rates of four-armed larvae at 96 h post fertilization were 87.89%±5.40% (control),55.11%±5.01% (OA1treatment), 40.44%±5.00% (OA2treatment) and 39.22%±0.51% (OA3treatment).Statistically signi ficant differences were observed between the control and all acidi fied-seawater treatments (Fig.5b, Table 4).
3.3 The impact of seawater acidi fication on larval morphology and spicule structure in G. crenularis
Most G. crenularis embryos exposed to current pH levels (i.e. control treatment) developed into the fourarmed larval stage with a good symmetrical shape, at 96 h after fertilization (Fig.6a (I, I′)). Larval spicules(endoskeleton) with obvious elements, such as body rods (BR) and post oral arms (PO), were also well developed in larvae from the control treatment. As pH declined, more abnormal larvae were observed with asymmetrically developed PO arms and elongated spicules (Fig.6a (II, II′, III, III′, IV, IV′)). The proportion of larvae that developed asymmetrically increased with decreasing pH in all acidi fied seawater treatments, and this trend was dose-dependent(Fig.6b, Table 5). Basic endoskeleton elements of developing pluteus larvae showed a trend towards increased length in acidi fied seawater (Fig.7a–c). The length of body rods ( LBR) and the distance between skeletal rods (SD) were longer in developing larvae that were exposed to the acidi fied treatments (Table 6). The length of the post oral arm ( LPO) in developing larvae showed a dose-dependent decreasing trend when ΔpHnbs>-0.4, compared with the control,however average LPOof larvae cultured in the OA3treatment (ΔpHnbs=-0.5) were longer than in larvae from the control group (Table 6).
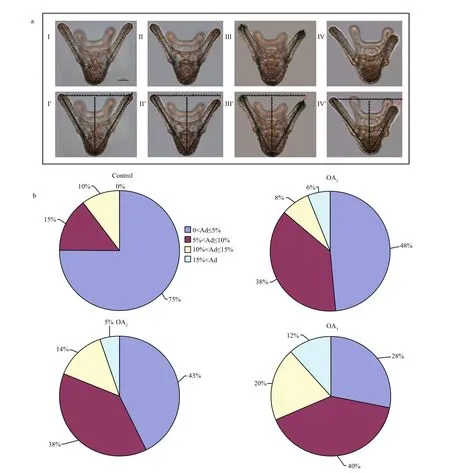
Fig.6 The impact of seawater acidi fication on larval morphology and symmetry in G. crenularis
The ultrastructure of the calci fied spicules of G. crenularis larvae from the control treatment were smooth, compact and unblemished (Fig.8a), but spicules in larvae reared in all acidi fied treatments had a corroded calci fied surface structure, which increased in a dose-dependent manner with decreasing pH (Fig.8b, c, d).
4 DISCUSSION
In this study we assessed the impact of CO2-induced seawater acidi fication on the sea urchin G. crenularis, focusing on impacts on fertilization,embryogenesis and the early pluteus stage. These key life-history stages are considered to be highly sensitiveto environmental stressors, such as pH, temperature and salinity, and these stages play a signi ficant role in determining population abundance, genetic diversity,distribution and resilience to disturbance (Grosberg and Levitan, 1992). As seawater acidi fication increased, there was a linear decrease in fertilization success and an increase in the percentage of abnormal fertilized eggs, suggesting that CO2-induced seawater acidi fication negatively affects fertilization in G. crenularis.
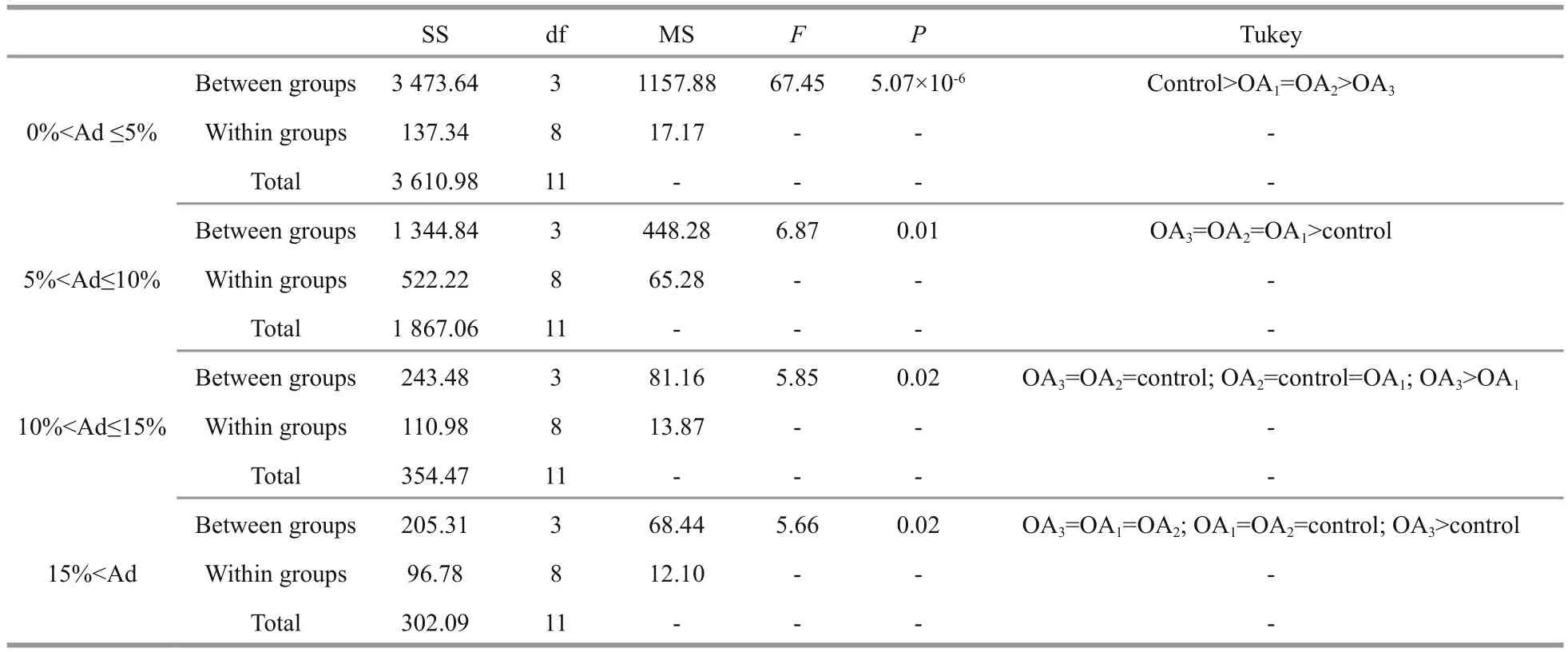
Table 5 One-way ANOVA results for the percentage of larvae with different degrees of asymmetry (Ad) in G. crenularis
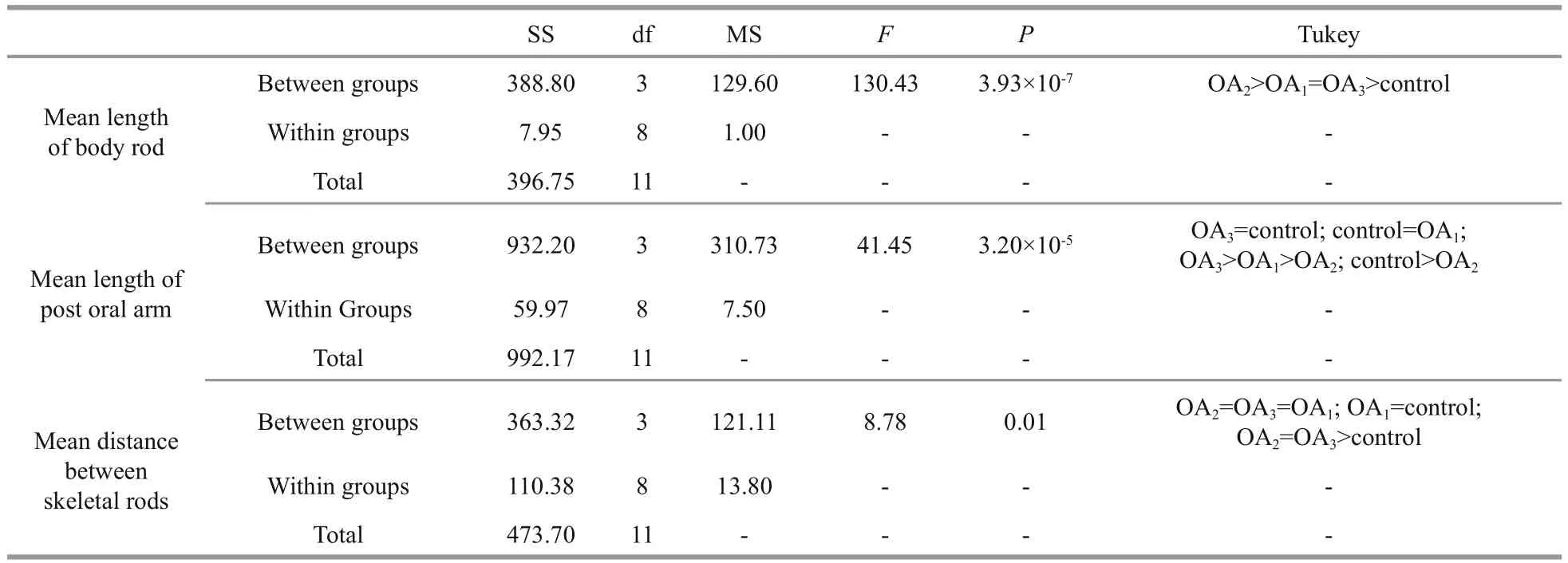
Table 6 One-way ANOVA results for the impact of seawater acidi fication on larval spicule elements in G. crenularis
Some studies report that fertilization is robust to the effects of seawater acidi fication in some sea urchin species (Dupont and Thorndyke, 2013; Zhan et al.,2016, 2017), however our observations support the hypothesis that the effects of seawater acidi fication on fertilization in echinoderms varies among species,with some being more tolerant than others (Byrne and Przeslawski, 2013; Dupont and Thorndyke, 2013).We found that the percentage of fertilized eggs in the OA2treatment (ΔpHnbs=-0.4 units) was 63.67% lower than in the control treatment (see Table S3). The percentage of eggs of the sea urchin Heliocidaris erythrogramma that developed into cleaving embryos at pH 7.7 (ΔpHnbs=-0.4 units) was 20.4% lower compared with the percentage of eggs that developed at a pH of 8.1 (Havenhand et al., 2008). Therefore, the fertilization process in G. crenularis seems more sensitive to CO2-induced seawater acidi fication,compared with H. erythrogramma. A recent study showed that CO2-induced seawater acidi fication affected sperm motility in the Australian sea urchin Centrostephanus rodgersii, by reducing mitochondrial activity (Schlegel et al., 2015). One possible explanation for the vulnerability of G. crenularis to seawater acidi fication during the fertilization process may be associated with decreased sperm motility at 30 min post-fertilization due to reduced mitochondrial activity. Further research needs to be carried out to clarify the molecular mechanism involved in the response of fertilization to seawater acidi fication in G. crenularis.
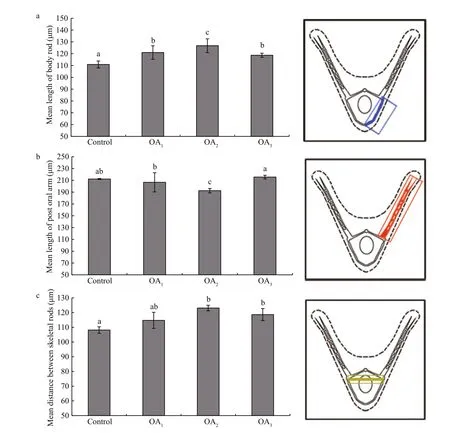
Fig.7 The impact of seawater acidi fication on larval spicule elements in G. crenularis
A dose-dependent reduction in the size of early cleavage stage G. crenularis embryos exposed to acidi fied seawater was observed in the current study,suggesting early developmental dysplasia. The hatching rate of blastulae and the survival rate of fourarmed pluteus larvae were negatively affected by seawater acidi fication in G. crenularis, and this is consistent with the results for other echinoderms including, Arachnoides placenta (Gonzalez-Bernat et al., 2013b), Odontaster validus (e.g. Gonzalez-Bernat et al., 2013a) and Patiriella regularis (Byrne et al.,2013a). The dose-dependent decline in the survival rate of four-armed larvae exposed to acidi fied seawater is consistent with results from our previous studies (Zhan et al., 2016, 2017). However, the survival rate of G. crenularis four-armed pluteus larvae, was more strongly affected by acidi fied seawater, than it was in the sea urchin Strongylocentrotus intermedius (Zhan et al., 2016).The survival rate of G. crenularis four-armed larvae was reduced by 37.34%±3.02% in the OA1treatment and by 54.09%±3.32% in the OA2treatment compared with the control group (see Table S3). In comparison,the survival rate of S. intermedius four-armed larvae was reduced by 15.81%±5.04% in the OA1treatment and by 29.90%±2.84% in the OA2treatment,compared with the control (Zhan et al., 2016).
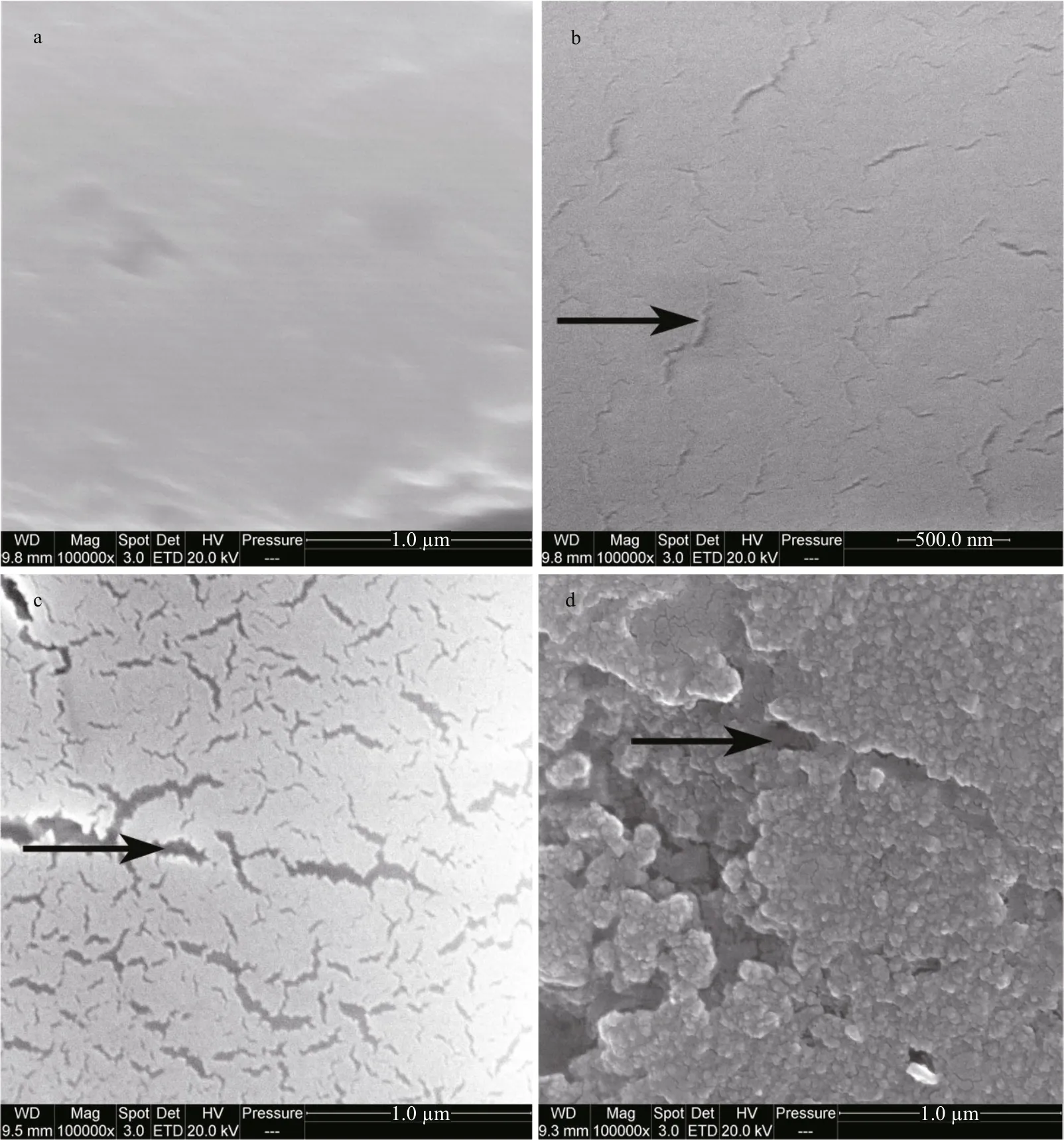
Fig.8 The impact of seawater acidi fication on larval spicule ultrastructure in G. crenularis
The proper development of morphology plays a critical role in helping sea urchin larvae carry out normal functions. However, in the current study we observed adverse effects on the morphological development of G. crenularis larvae cultured in acidi fied seawater, characterized by impaired body symmetry and slightly elongated and corroded spicules. This finding is consistent with the results we obtained in a previous study on the sea urchin Hemicentrotus pulcherrimus (Zhan et al., 2017). It seems likely that abnormal morphological development will affect the ability of G. crenularis larvae to carry out normal functions. Impaired larval symmetry caused by imbalanced development of PO arms in G. crenularis might affect the stability of larvae in flowing water and the ability to capture food and to swim, reducing the chance of long-term survival and recruitment to the adult population. The elongation of spicules suggests an increase in calcium carbonate assimilation. This result differs from our study on S. intermedius, as four-armed larvae of this species developed shortened spicules in response to decreased pH (Zhan et al., 2016). This suggests interspeci fic phenotype plasticity in sea urchins when exposed to extracellular acidosis. We also observed that the protective spicules of G. crenularis larvae were corroded and elongated in the seawater acidi fiedtreatments. This may weaken the calci fied structures and could affect the normal function of larval spicules.We postulate that G. crenularis larvae may not be able to fully compensate for the damage to calci fied structures induced by seawater acidi fication.
5 CONCLUSION
In this study, we found that CO2-induced seawater acidi fication has a negative impact on the early development and calci fication of G. crenularis larvae.Morphological data collected in this study support the hypothesis that there are inter-speci fic differences in the response of sea urchins exposed to decreased seawater pH. Additional studies on the effects of acidi fied seawater on juveniles, adults, and on generations of G. crenularis should be carried out.Work to clarify the role of bio-molecular and adaption mechanisms, such as alteration in gene expression,single-nucleotide polymorphism (SNPs), microRNAs and proteomics should also be conducted to further understand the impacts of ocean acidi fication.
6 ACKNOWLEDGEMENT
We are very grateful to the reviewers for their helpful comments.
7 AUTHORS’ CONTRIBUTION
Y. Y. Z. and Y. Q. C. conceived and designed the experiments. The experiments were performed by W.B. H., M. B. L., L. Z. D. and W. J. Z. Data were analyzed by Y. Y. Z., Y. Q. C. and C. L. The paper was written by Y. Y. Z. and W. B. H. All authors read and approved the manuscript.
猜你喜欢
杂志排行
Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- Recent insights into physiological responses to nutrients by the cylindrospermopsin producing cyanobacterium,Cylindrospermopsis raciborskii*
- Response of Microcystis aeruginosa FACHB-905 to different nutrient ratios and changes in phosphorus chemistry*
- In fluence of light availability on the speci fic density, size and sinking loss of Anabaena flos- aquae and Scenedesmus obliquus*
- Application of first order rate kinetics to explain changes in bloom toxicity—the importance of understanding cell toxin quotas*
- Regime shift in Lake Dianchi (China) during the last 50 years*
