生物相溶性多孔炭-香豆素复合材料的制备及其细胞成像和靶向给药
2018-05-02YallappaShoriyaAruniAbdulManafGurumurthyHegde
S. Yallappa, Shoriya Aruni Abdul Manaf , Gurumurthy Hegde
(1.BMS R and D Centre, BMS College of Engineering, Bangalore560019, Karnataka, India;2.Department of Chemistry, Gokhale Centenary College, Ankola, Uttara Kannada581314, India;3.Faculty of Industrial Sciences and Technology, Universiti Malaysia Pahang26300, Gambang, Kuantan, Malaysia)
1 Introduction
Nanostructured materials are extensively used in delivering drugs, genes, proteins, biomolecules and florescent agents for the simultaneous diagnosis and treatment of various types of diseases[1,2]. Recently, targeted drug delivery (ca: active targeting) strategy was devised to improve the delivery efficiency through conjugating with specific receptors and biomolecules which over-expressed on the diseased cells[3,4]. Moreover, such conjugated nano-scaled material delivery into the tumor model system has led to a variety of benefits such as enhanced tumor targeting, minimum amount of drug to the targeted site for an essential period of time through, elevating tumor cell uptake (invitro) proficiently and accurately and minimized side effects (invivo)[5-7]. Nowadays, a variety of nanomaterials such as gold[8,9], silica[10], carbon nanotubes[11,12], semiconductor quantum dots[13], magnetic nanoparticles[14]and polymer nanocarriers[15]are being actively explored for the purpose of cell imaging and specific targeted intracellular drug delivery. However, many of these materials have properties that considerably reduce their therapeutic efficacy, including poor photostability and solubility in aqueous media, low drug loading capacity, poor biodistribution and a lack of target selectivity[16-18].
Among the various types of nanomaterials[8-15], nanoporous carbons (NPCs) gain much interest in imaging and drug delivery applications owing to their high surface area, chemical stability, good affinity to biomolecules, biocompatibility and generally nil toxicity[19-21]. Moreover, it is observed that NPCs displays remarkable conjugation capabilities owing to their high surface area, which allows them to bind a variety of biomolecules through covalent bonds, transport them across the cell membranes and release them effectively in cancer cells. This improves clinical outcomes and comprises the next generation of multifunctional nanomedicine[22]. Recently, Wang et al.[23]have reported hollow NPCs as a novel platform for delivering the anticancer drug and generating additional cellular reactive oxygen species under near infrared laser irradiation. These irradiated NPCs sufficiently catalyzed persistent free radicals to produce a large number of heat shock factor-1 protein homotrimers, thereby suppressing the activity and function of resistance-related genes.
NPCs in cell imaging and drug delivery applications are mainly produced by carbonization of different liquid or gaseous hydrocarbons (cyclohexane, xylene, anthracene etc.), petroleum products, graphite powder and polymers, et al.[24,25]. However, these chemicals are becoming scarce and have a negative impact on the environment because they often require harmful reagents and energy-demanding synthetic conditions. Moreover, the materials made with these precursors are not well controlled morphologically and hence it is not suitable for many applications including biomedical and pharmaceutical applications. Thus, there is a crucial need to explore environmentally benign, morphologically controlled and economically feasible methods for large scale production of advanced and more sustainable NPCs. The use of bio-renewable abundant carbon precursors that are not in competition with food suppliers, such as diverse biomasses (e.g. plant biomass, starch, carbohydrates, glucose, lignin, hemicellulose, proteins, et al.) and biomass derivatives (e.g. hydroxylmethylfurfural and furfural) to produce multifunctional NPCs has attracted a considerable research attention. Among them, plant biomass derived carbon nanomaterials have good prospects in tissue engineering scaffolds, tumor cell detection and cell tracking, controlled release of drug and cellular imaging applications[26,27]. Recently, our group[28]and other research group[22]reported green NPCs derived from bio-waste and agricultural waste, respectively, as novel drug carriers and florescent probes owing to their less toxicity and excellent intrinsic photoluminescence properties. Even though NPCs do not conjugate any targeting moieties on their surfaces, they are highly biocompatible and stable in aqueous media, allowing their use in biomedical applications[21]. Based on these observations, we synthesized spherical-shaped NPCs with a diameter of <50 nm using natural bio-waste of oil palm leaves (OPL) with a single step pyrolysis method. The obtained NPCs were conjugated with coumarin-6 (C-6), a hydrophobic florescent dye, in order to enhance the florescent property and to develop an efficient imaging scaffold for the purpose of anticancer therapy. This strategy would be an ideal to engineer ‘advanced’ versatile NPCs that are proficient of performing biological actions such as bioimaging, biosensing and targeted drug delivery by a simple fabrication and conjugation strategy.
In this study, we describe the design of an advanced green solution for the fabrication and conjugation of NPCs with C-6 for cellular imaging and its delivery to cancer cells (Scheme 1). High quality NPCs were prepared from bio-inspired renewable source of OPL using our simple single-step pyrolysis technique at 550 ℃, which exhibited excellent size uniformity, micro-porosity, high photostability and low cytotoxicity. In our simple method high dispersion and high drug loading capacity were achieved without adding any external hydrophilic agents since their functional groups were hydrophilic. This offersinvitrotherapeutic NPCs (green NPCs conjugated with C-6: NPCs-C-6) suitable for cell imaging and drug delivery. We report here a facile and simple method for the synthesis of NPCs-C-6 that can be used for cell imaging and deliver efficiently drugs to cancer cells, A-375 (Melanoma, human), N2A (neuroblastoma cells, human) and MDCK (normal epithelial cells, human). These have provided new materials for simultaneous therapeutic targeting and diagnostic imaging.
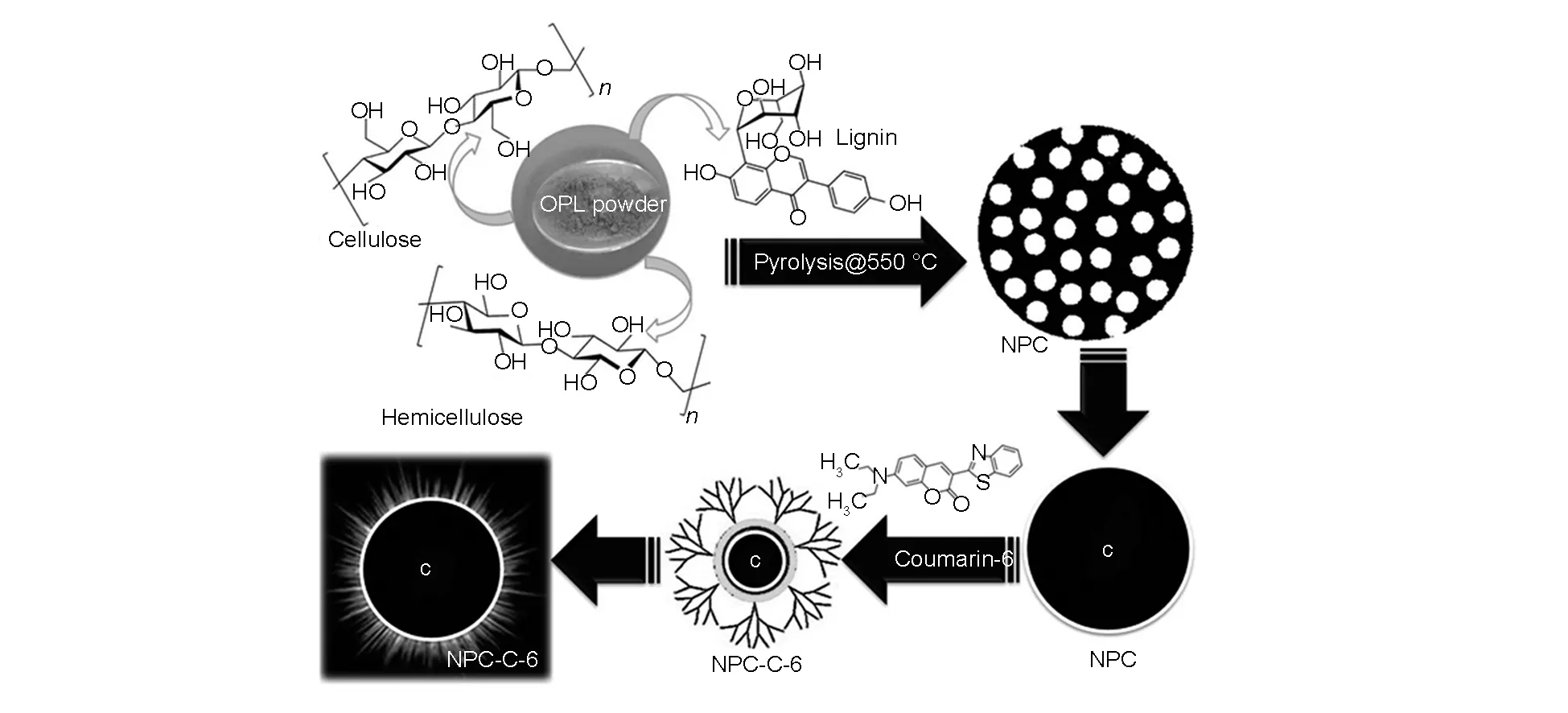
Scheme 1 Synthesis of biocompatible NPCs and its bio-conjugation process with C-6 dye.
2 Experimental
2.1 Materials and methods
The dry OPL was collected from nearby local oil palm plantation in Malaysia. The central vein (midrib) of OPL was separated and dried in hot air oven at 60 ℃ for about two days to remove moisture. The dried OPL was ground into fine powder using an ultra grinder (Retsch, ZM 200, Germany) at 120 00 rpm. In order to attain the uniform sized raw powder, we further sieved at 62 μm using a laboratory sieving machine. This was used as a precursor for the synthesis of NPCs under pyrolysis in a tube furnace (Nabertherm, EW-33334-36) at 600 ℃ for 2 h (heating rate of 5 ℃/min) with a continuous flow of nitrogen (150 mL/cm3). The obtained slurry was washed with 1mM HCl, followed by distilled water to remove ash or any other impurities.
2.2 Surface modification and conjugation of NPCs with C-6 molecule
As-prepared NPCs were pretreated with a mixture of sulfuric acid and nitric acid (3∶1v/v) for a few hours to remove any metal ions and also to impart the functional groups to improve their dispersion in aqueous media. The resulting surface-modified NPCs were collected by centrifugation at 3 000 rpm for 30 min and dried at 40 ℃ for 24 h.
In the conjugation of NPCs with C-6, 10 mg of each surface-modified NPC and C-6 was taken in a beaker containing 20 mL of distilled water. The reaction mixture was kept for stirring under nitrogen atmosphere for 24 h. After completion of the reaction, NPC-conjugated C-6 was collected by centrifuging at 3 000 rpm for 30 min. The free C-6 molecules were removed by using a 3000 kDa dialysis tube in phosphate buffer saline at neutral pH. The post dialyzed sample was further centrifuged at 3 000 rpm for 30 min. The nanoparticles conjugated with florescent dye obtained here were washed with distilled water and dried in hot air oven at 40 ℃ for 24 h to obtain the samples in a powder form.
2.3 Characterization
The powder X-ray diffraction (XRD, Rigaku/Miniflex II) patterns of NPCs were recorded using a ‘X’PERT-PRO XRPD (CuKα, k=0.154 06 nm) with a scanning rate of 1°/min ranging from 10 to 80°. The structural morphology was performed by field emission electron microscopy (FESEM, JEOL/JSM-7 800 F) and transmission electron microscopy (TEM, JEOL/JSM 1230). The energy dispersive X-ray (EDX) analysis was used to know the elemental compositions. Fourier transform infrared (FT-IR, Bruker-TENSOR 27) spectra were recorded at a spectral resolution of 4 cm-1in KBr pellet to know the conjugation of C-6 dye to NPCs. The zeta potential was measured using a Malvern Mastersizer 2000 (Zetasizer Nano ZS90, Malvern Instruments Ltd., UK) to observe the suspension ability and surface charge of the materials in colloidal systems. The Raman spectrum was recorded using HORIBA Scientific Raman spectroscopy. The fluorescence images of the treated and untreated cell lines with NPCs were measured by a Nikon Eclipse Ti microscope. All measurements were carried out in room temperature.
2.4 Cell culture
The different cell linens namely MDCK (ATCC® CCL-34TM), A-375 (ATCC® CRL-1619TM) and N2A (ATCC® CCL-131TM) were maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin streptomycin at 37 ℃ in a humidified atmosphere with 5% CO2. The medium was changed every two days and the cells were separated by trypsinization before reaching confluence. The stock cells were maintained in 25 cm2and 75 cm2tissue culture flasks.
2.5 Cytotoxicity assay
The cytotoxicity of conjugated nanomaterial was evaluated by using the MTT (colorimetric) method as follows[22]. The selected cell lines were seeded in 96-well plates at a density of 2.0 × 104cells per well. After incubation for 24 h, NPCs, free C-6 and NPCs-C-6 complex were added to the cells in a phosphate buffer saline (PBS) at different concentrations (0-250 μg/mL) and kept for incubation at 37oC in the humidified 5% CO2atmosphere for 24 h. At the termination of culture, cells were rinsed with the PBS solution and 20 μL of MTT solution (5 mg/ml) was added to each well. The cells were cultured for another 4 h. Then, 100 μL of dimethyl sulfoxide was added to each well to dissolve formazan crystals and the absorbance of the solutions was monitored at 570 nm, with a reference at 630 nm on a microplate reader (Infinite M200 Nanoquant, Tecan). Untreated cells were used as controls and the experiments were tested in triplicate. The percentage of cell viability was calculated by the following equation:
Cell viability (%) = [ (ASample-ABlank) /
(AUntreated-ABlank)] × 100
2.6 Cell imaging
The test cell lines as illustrated above were seeded on glass cover slips in twelve-well culture plates (2.0 × 104cells per well) and allowed to stick for overnight. Then the cells were kept for incubation with serial concentrations of test samples in 3 mL of the serum-free DMEM medium in a 5% CO2atmosphere for 24 h. Subsequently, the cells were washed with the PBS solution to remove excess samples and dead cells. For cell imaging, the cells were treated with 1.0 mL 4% paraformaldehyde solution for a few minutes at 4 ℃. Finally, nuclei were stained by using 2-(4-amidinophenyl)-6-indole carbamidine dihydrochloride in 10% glycerol for 20 min in the dark. The cell images were observed using a Nikon Eclipse Ti fluorescence microscope.
3 Results and discussion
3.1 Formation of bio-inspired NPCs
OPL are waste lignocellulose biomass from oil palm industries, which are abundant in South-East Asia[29]. OPL can be used as a carbon precursor for the development of orderly formed synthetic carbon, so called waste to wealth approach owing to the presence of cellulose (44.5 wt%), hemicelluloses (47.7 wt%), pectin and lignin (27.35 wt%) in OPL[30]. Thus, the present work demonstrates the synthesis of NPC by pyrolysis at 550 ℃. The powder XRD pattern of the obtained NPC is shown in Fig. 1a. The prominent peaks observed at 2θ=24.4° and 41.1° correspond to the (002) and (101) planes of the face-centered cubic (FCC) structure of graphite (ICDD-10750410) with an interlayer d-spacing of 0.3 352 nm. The pyrolyzed carbon is found to be pure without any impurities and matches the existing literature on graphite[31]. The sharpness of peaks indicates the presence of crystalline nature of the obtained NPC[31,32]. The average crystalline size of the NPC is calculated to be about 35 nm using the Scherrer’s equation (d=Kλ/βcosθ, whereKis a shape factor between 0.9 and 1.1,λ(CuKα)=0.1 542 nm,βandθare the full width at half maximum and the position of the prominent line).

Fig. 1 (a) Powder XRD pattern and (b) Raman shifts of as-obtained NPCs.

3.2 Conjugation of NPC with C-6 dye
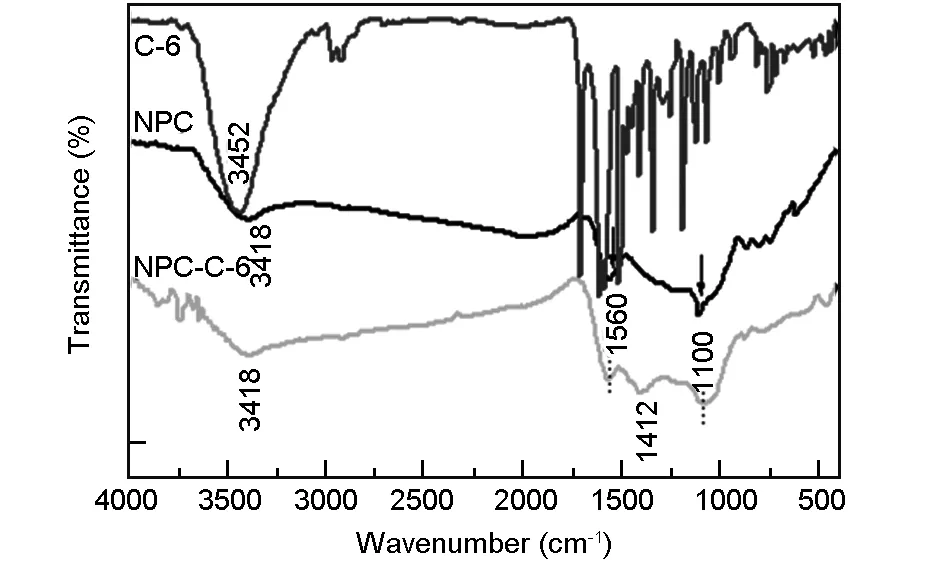
Fig. 2 FTIR spectra of C-6, NPCs (before conjugation), and NPCs-C6 (after conjugation).
Fig. 3 shows the FESEM images and corresponding EDAX spectra of NPC before and after conjugation with the C-6 dye molecule.
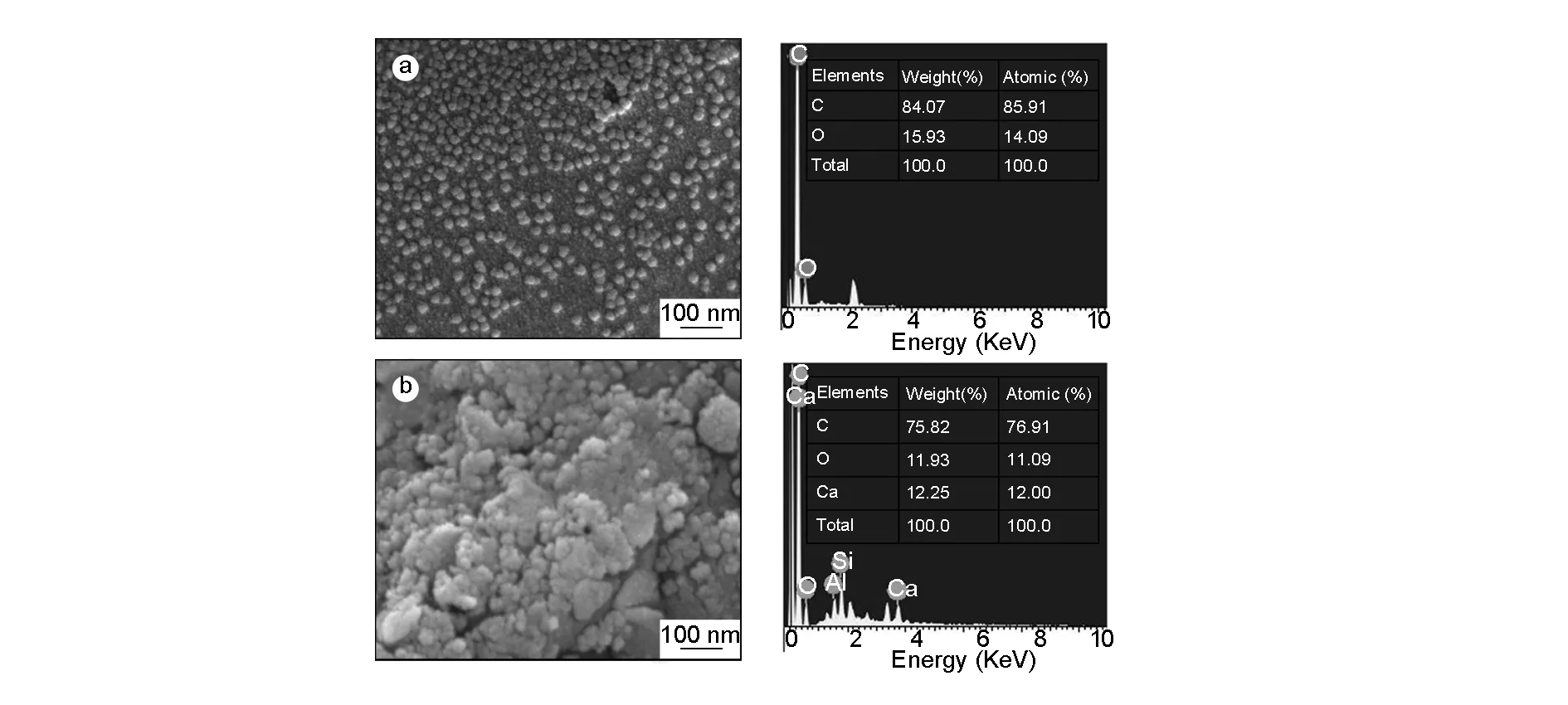
Fig. 3 FESEM images of NPCs (a) before, (b) after conjugation with C-6, and their corresponding EDX analysis.
It is clear that the NPC is of spherical in shape and uniform in size distribution with a diameter range from 35-45 nm in both t case, which is ideal for cell imaging and drug delivery applications. The promising drug delivery into diseased sites can be achieved through spherical-shaped nanoparticles with a sub-50 nm diameter[35]. On carful observation we can see that a thin layer of foam (scum) is adsorbed on the surface of the conjugated NPC, which is ascribed to anchored dye molecule.
The spherical shape with a uniform size distribution of the NPC is also evident from TEM analysis (Fig. 4). The average particle size is ~30-50 nm as obtained from corresponding histogram (Fig. 4b). No other significant morphology difference is observed before and after conjugation with C-6 dye molecule. The elemental composition from EDAX spectra shows about 82% of carbon and the remaining minor amounts of O and Ca due to conjugation of drug molecule. Such a conjugation of NPC with a florescent dye helps to protect them from agglomeration and improves the florescent properties of nanoparticles, indicating the suitability in drug delivery and cell imaging applications.
The stability of nanoparticles in aqueous media is very significant for exploring their biomedical and pharmaceutical applications.The nanoparticles are typically stabilized by adding surfactants and polymers under different conditions. However, in this study even though any such external agents are not used, a significant stability has been achieved. Zeta potential is used as an index of the magnitude of electrostatic interaction between colloidal particles, which indicates the degree of repulsion between adjacent and similarly charged particles in a dispersion solution. Fig. 5 shows the zeta potential of NPC (Fig. 5 a-1) and the NPC conjugated with-C-6 dye (Fig.5 a-2). In this study, a high and stable dispersion in aqueous media is obtained before and after conjugation of NPC with C-6 dye molecule. However, the measured zeta potential peak values are -30.5 mV and -31.5 mV before and after conjugation, respectively. This is an indication of a stable dispersion, especially in the physiological pH range employed here. Generally, the zeta potential values ranging between -30 to+30 mV were believed as stable. The NPC was also tested in an aqueous solution along with fluorescent coumarin C-6 dye, where the suspension of NPC in the aqueous solution is completely transparent (Fig.5 b-1) whereas the conjugated NPC has its original color due to the presence of fluorescent dye (Fig.5 b-2). This gives a preliminary idea about the suitability of the present NPC for cell imaging application. Thus, the prepared NPC is stable from electrostatic consideration, which is vital for their application in biomedical field such as drug delivery[27].

Fig. 4 TEM images of NPCs (a) before, (b) after conjugation with C-6, and (a-1,b-1) their corresponding particle size distribution histograms.
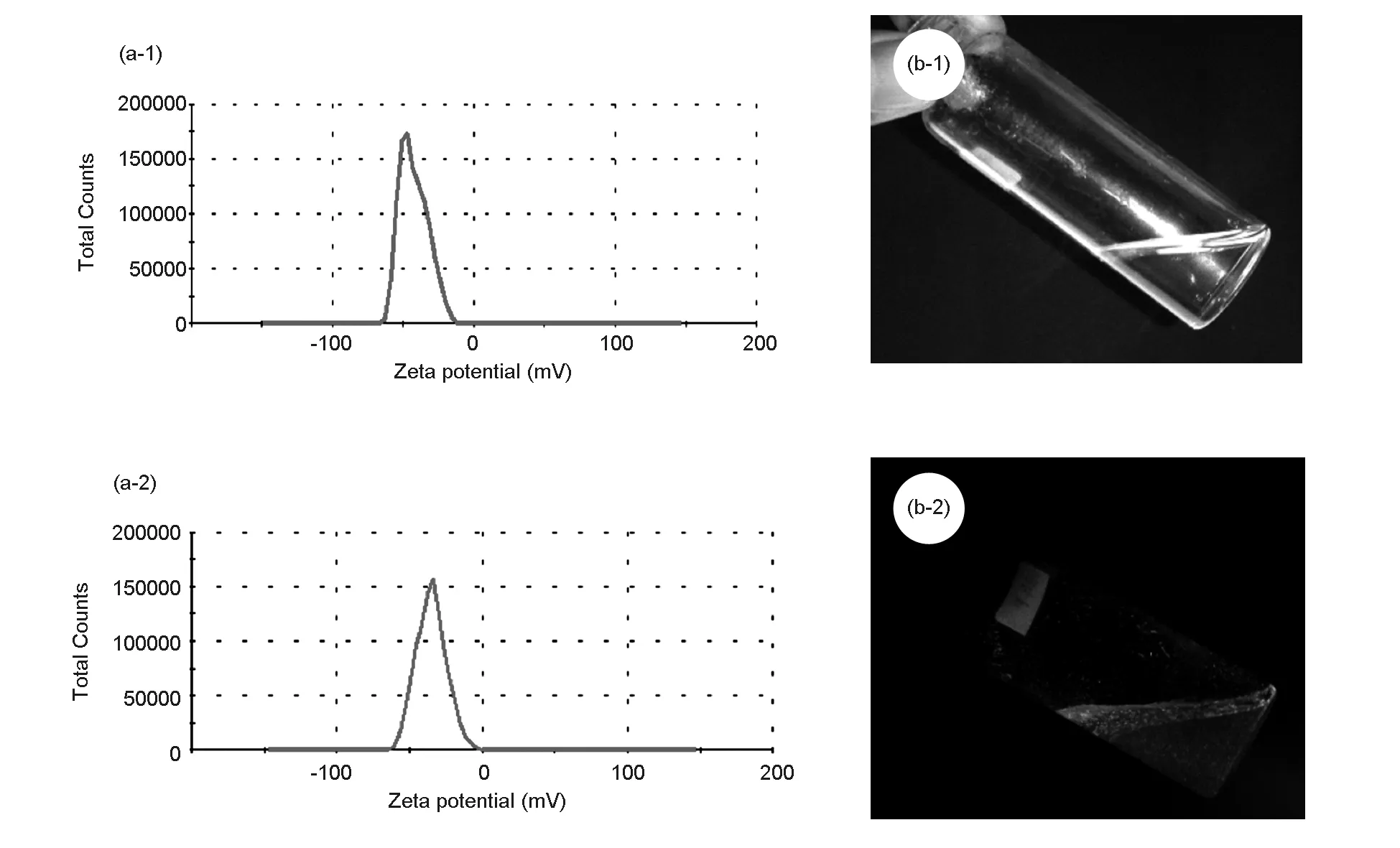
Fig. 5 Zeta potentials of NPCs, (a-1) before, and (a-2) after conjugated with C-6 dye. Photographs of aqueous solutions of NPCs, (b-1) before and (b-2) after conjugated with C-6 dye.
3.3 In vitro cytotoxicity and cellular uptake
The biocompatibility of a nanomaterial is a significant issue in biomedical and pharmaceutical applications. In order to verify the biocompatibility and cytotoxicity of free C-6 and the NPC-C-6, they were investigated by the MTT assay as follows. The normal cells (MDCK) and cancer cells (A-375 and N2A) were treated with free C-6, and the NPC-C-6 complex. Fig. 6 shows the impact of free C-6, and NPC-C-6 on normal and cancer cells after incubation for 24 h with different concentrations, 25-250 μg/mL. The cell viability results reveal that different cells treated with before and after conjugated nanomaterials exhibit a dosage dependent and time-dependent behavior. However, the as obtained NPC has no obvious cytotoxic effect on normal and cancer cells (recorded values are negligible and data not shown here), which indicates an excellent biocompatibility of NPC. The low cytotoxicity of the nanoparticles demonstrates that NPC meets the requirements of potential biological applications. Nevertheless, it is worthy exploring that the cytotoxic effect of the NPC after the modification with the C-6 flurocent dye is enhanced, as indicated in Fig. 6.
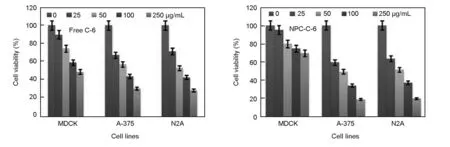
Fig. 6 In vitro cytotoxicity of free C-6 and NPC-C-6 against different cell lines. Data are represented as mean ± SD, n = 3.
For comparison, the cytotoxic effect of C-6 dye and NPC-C-6 treated on normal and cancer cells is found to be significantly high at different concentrations (0-250 μg/mL)(Table 1).
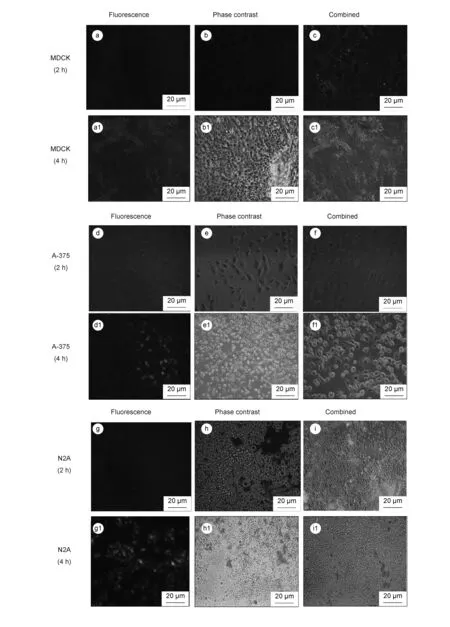
Fig. 7 Fluorescence images of MDCK (a, a1-c, c1), A-375 (d,d1-f,f-1) and N2A (g, g1-I, i1) cells incubated with NPC-C-6 NPs for 2 h and 4 h.
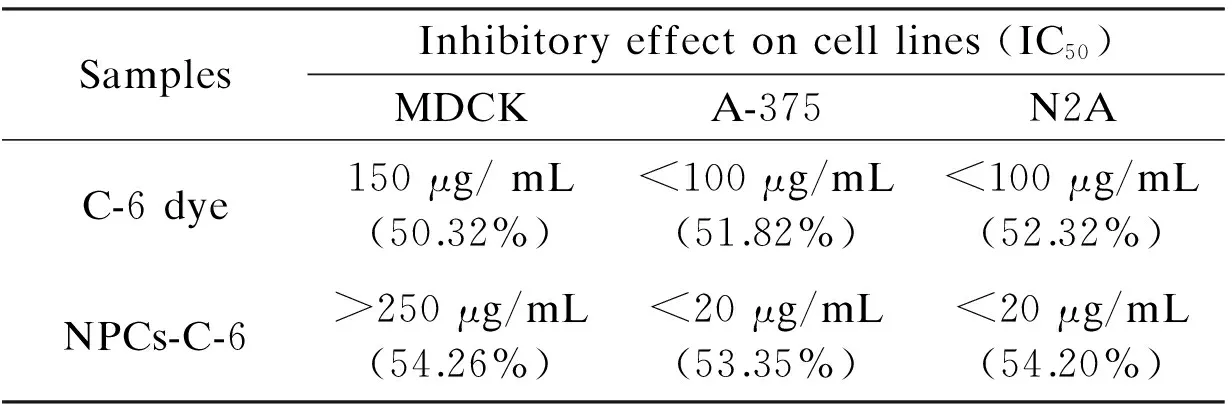
Table 1 Growth-inhibitory effect (after 24 h) of free C-6 and NPCs-C-6 complex.
For example, the survivability of cells is found to be 46% for normal cells and 36% for cancer cells at a high dose (250 μg/mL) of C-6 dye, which is generally considered as a high toxicity for all these tested cell lines. The IC50values of C-6 is found to be 150 μg/mL for normal cells and <100 μg/mL for cancer cells after a 24 h exposure, indicating the potential toxicity of dye molecules in chemotherapy. The cytotoxic effect of the NPC-C-6 is considerably high for cancer cells compared with free C-6 dye. For instance, the cell viability is found to be 70% for normal and ~16%-18% for cancer cells at high concentrations. The IC50value of NPC-C-6 complex on normal and cancer cells is found to be >250 μg/mL and <20 μg/mL respectively, indicating the biocompatibility for normal and highly toxic for cancer cells even at low doses. The biocompatibility for normal cells may be due to the impact of targeting agents. Recently Liu et al.[4]have reported that NPCs are important materials for drug carrier and greatly useful in tumor cell detection as well as cellular imaging applications. Moreover, Cheng et al.[17]and Yu et al.[18]also have reported the toxicity of NPCs functionalized with a variety of biomolecules on various cancer cells. Although, more detailed of cell interaction studies on NPCs conjugated with florescent dye are required for revealing the precise mechanism. There are many controversy results in the literatures concerning the function of targeting molecules. A large number of studies have revealed an increased localization in the tumor site when nanoparticles are conjugated with specific tumor targeting agents, while some studies have revealed no significant difference between targets and nanoparticles modified and unmodified[36]. In an another study by Kirpotin et al, it is reported that NPs modified targeting molecules certainly increase the localization into tumor cells[37]. However, fluorescence microscopy is a powerful technique to observe the cellular uptake of nanoparticles at different incubation times. Thus, in order to confirm the efficacy of the NPC-C-6 complex to enter the live cells, cellular uptake experiment was performed on normal cells (MDCK) and cancer cells (A-375 and N2A). After 2 h and 4 h incubations at 37 ℃, the cells treated with nanoparticles were washed with PBS and observed by a fluorescence microscope to know cellular distribution of the nanoparticles (Fig. 7).
The fluorescent images show that nanoparticles are efficiently taken up and/ or internalized into cells and light up the cells. To know the effects of incubation time and concentration, the fluorescence intensities of cellular nanoparticles were recorded. These results (Fig.7) reveal that fluorescence intensities gradually increase in cells along with the incubation time and concentration of nanoparticles, thus indicating that NPC-C-6 has a high capacity for cell uptake/internalization. For instance, when the cells were treated for 2 h, the cellular uptake of NPC-C-6 complex was not significant. However, after 4 h treatment, the green fluorescence is observed around the nucleus of the cells in all the cases, indicating nanoparticles localization within cells. The obtained results in this study are consistent with recent studies of photoluminescent NPCs from cyanobacteria[38]. The size of the nanoparticles might be different in cellular uptake of nanoparticles in each type of the cells. The particles with a smaller diameter of (<100 nm) have more efficient cellular uptake than those with a larger diameter. Besides, different types of cells have different ways of cellular uptake mechanisms[35]. More investigations are required to understand the exact mechanism of cellular uptake of nanoparticles in different types of cells.
4 Conclusions
In summary, a novel class of biocompatible NPC ranging from 35-40 nm was successfully prepared by using bio-renewable source of OPL by the pyrolysis method and conjugated with C-6 fluorescence dye for cellular imaging and drug delivery to cancer cells. The uniform size distribution, large surface area and hydrophilic surface of NPC make it excellent in biocompatibility and dispersibility in aqueous media. The NPC was effectively conjugated with C-6 dye, forming a NPC-C-6 complex. Such a complex is examined for cytotoxicity and biocompatibility against normal cells (MDCK) and cancer cells (A-375 and N2A) in comparison with free C-6 dye. The dye conjugated NPC has a low toxicity for normal cells and high toxicity for cancer cells. Furthermore, the NPC conjugated with C-6 could be easily internalized into the tested cancer cells and demonstrates a good biocompatibility. We foresee that the obtained NPC has much potential to be used in different biomedical applications such as cell imaging and target sensing of cancer cells.
We wish to thank the director R and D Centre and the Principal BMS College of Engineering, Bangalore for their constant support in encouraging this research work. We also thank Dr. Partha Roy and Dr.Anuj Kumar for helping in initial experiments.We acknowledge DST-Nanomission, Govt of India for providing the grant with grant number SR/NM/NT-1026/2017.
[1] Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs[J]. Cancer Res, 1986, 46: 6387-92.
[2] Jain R K. Delivery of molecular and cellular medicine to solid tumors[J]. Adv Drug Deliv Rev, 2001, 46: 149-68.
[3] Mickler F M, Möckl L, Ruthardt N, et al. Tuning nanoparticle uptake: live-cell imaging reveals two distinct endocytosis mechanisms mediated by natural and artificial EGFR targeting ligand[J]. Nano Lett, 2012, 12: 3417-23.
[4] Liu Y, Lu W. Recent advances in brain tumor-targeted nano-drug delivery systems[J]. Expert Opin Drug Deliv, 2012, 9: 671-86.
[5] Ashley C E, Carnes E C, Phillips G K, et al. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers[J]. Nat Mater, 2011, 10: 389-97.
[6] Florence AT. Targeting nanoparticles: the constraints of physical laws and physical barriers[J]. J Control Release, 2012, 164: 115-24.
[7] Agarwal S, Sane R, Oberoi R, et al. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain[J]. Expert Rev Mol Med, 2011, 13: 17.
[8] Brown SD, Nativo P, Smith Jo-Ann, et al. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin[J]. J Am Chem Soc, 2010, 132: 4678-84.
[9] Yallappa S, Manjanna J, Dhananjaya BL, et al. Phytosynthesis of gold nanoparticles using Mappia foetida leaves extract and their conjugation with folic acid for delivery of doxorubicin to cancer cells[J]. J Mater Sci: Mater Med, 2015, 26: 235-47.
[10] Barbe C, Bartlett J, Kong L, et al. Silica particles: A novel drug-delivery system[J]. Adv Mater, 2004, 16: 1959-66.
[11] Kunal B, Sourav P M, Audrey G, et al. Biological interactions of carbon-based nanomaterials: From coronation to degradation[J]. Nanomedicine, 2016 , 12(2): 333-351.
[12] Feng Y, Lee K, farhat H, et al. Current ON/OF ration enhancement of FETs with bundled CNTs[J]. J Appl Phys, 2009, 106(10): 104505-09.
[13] Chun X G, Jiale X, Bin W, et al. A new class of fluorescent-dots: long luminescent lifetime bio-dots self-assembled from DNA at low temperatures[J]. Sci Rep, 2013, 3, 2957: 1-6.
[14] Mou X, Ali Z, Li S, et al. Applications of magnetic nanoparticles in targeted drug delivery system[J]. J Nanosci Nanotechnol, 2015, 15(1): 54-62.
[15] Xiao K, Luo J, Li Y, et al. PEG-oligocholic acid telodendrimer micelles for the targeted delivery of doxorubicin to B-cell lymphoma[J]. J Controlled Release, 2011, 155: 272-81.
[16] Jaeyun K, Lan C, Dmitry S, et al.Targeted delivery of nanoparticles to ischemic muscle for imaging and therapeutic angiogenesis[J]. Nano Lett, 2011, 11:694-700.
[17] Cheng Z 1, Al Zaki A, Hui J Z, et al. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities[J]. Science, 2012, 338: 903-10.
[18] Yu C, Hangrong C, Deping Z, et al. Core/shell structured hollow mesoporous nanocapsules: a potential platform for simultaneous cell imaging and anticancer drug delivery[J]. ACS Nano, 2010, 4: 6001-13.
[19] Shihui W, Hui L, Hongdong C, et al. Drug delivery: targeted and pH-responsive delivery of doxorubicin to cancer cells using multifunctional dendrimer-modified multi-walled carbon nanotubes[J]. Adv Healthcare mater, 2013, 2: 1267-76.
[20] Xinxing M, Huiquan T, Kai Y, et al. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging[J]. Nano Res, 2012, 5: 199-212.
[21] Nanda G S, Hongqian B, Yongzheng P, et al. Functionalized carbon nanomaterials as nanocarriers for loading and delivery of a poorly water-soluble anticancer drug: a comparative study[J]. Chem. Commun, 2011, 47: 5235-37.
[22] So Y P, Hyun U L, Eun S P, et al. Photoluminescent green carbon nanodots from food-waste-derived sources: Large-scale synthesis, properties, and biomedical applications[J]. ACS Appl Mater Interfaces, 2014, 6: 3365-70.
[23] Wang L, Sun Q, Wang X, et al. Using hollow carbon nanospheres as a light-induced free radical generator to overcome chemotherapy resistance[J]. J Am Chem Soc, 2015, 137 (5): 1947-55.
[24] Zhang X B, Tong H W, Liu S M, et al. An improved Stöber method towards uniform and monodisperse Fe3O4@C nanospheres[J]. J Mater Chem A, 2013, 1: 7488-93.
[25] Pol VG, Motiei M, Gedanken A, et al. Carbon spherules: synthesis, properties and mechanistic elucidation[J]. Carbon, 2004, 42: 111-116.
[26] Pei-Ying L, Chiung-Wen H, Mei-Lang K, et al. Eco-friendly synthesis of shrimp egg-derived carbon dots for fluorescent bioimaging[J]. J Biotech, 2014, 189: 114-19.
[27] Daeun K, Yuri C, Eeseul S, et al. Sweet nanodot for biomedical imaging: carbon dot derived from xylitol[J]. RSC Adv, 2014, 4: 23210-13.
[28] Manar SAA, Roy P, Sharma K V, et al. Catalyst-free synthesis of carbon nanospheres for potential biomedical applications: waste to wealth approach[J]. RSC Adv, 2015, 5: 24528-33.
[29] Rafatullah M, Ahmad T, Ghazali A, et al. Oil palm biomass as a precursor of activated carbons: a review[J]. Crit Rev Environ Sci Technol, 2013, 43(11): 1117-61.
[30] Hashim R, Nadhari WNAW, Sulaiman O, et al. Characterization of raw materials and manufactured binderless particles board from oil palm biomass[J]. Mater Des, 2011, 32: 246-254.
[31] Wang J T, Chen C, Wang E, et al. A new carbon allotrope with six-fold helical chain in all sp2bonding networks[J]. Sci Rep, 2014, 4: 4339-44.
[32] Krishnamurthy G, Namitha R. Synthesis of structurally novel carbon micro/nanospheres by low temperature-hydrothermal process[J]. J Chil Chem Soc, 2013, 58(3): 1930-33.
[33] Panagiotis T, Thomas F F, Peter S. Carbon as catalyst and support for electrochemical energy conversion[J]. Carbon, 2014, 75: 5-42.
[34] Galeener F L, Sen P N. Molecular-dynamics study of a three-dimensional one-component model for distortive phase transitions[J]. Phys Rev B, 1978, 17: 1928.
[35] Hussain N, Jaitley V, Florence AT. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics[J]. Adv Drug Deliv Rev, 2001, 50: 107-42.
[36] Choi C H, Alabi C A, Webster P, et al. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles[J]. Proc Natl Acad Sci USA, 2010, 107: 1235-40.
[37] Kirpotin D B, Drummond D C, Shao Y, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models[J]. Cancer Res, 2006, 66: 6732-40.
[38] Hyun U L, So Y P, Eun S P, et al. Photoluminescent carbon nanotags from harmful cyanobacteria for drug delivery and imaging in cancer cells[J]. Sci Rep, 2014, 4: 4665-72.
