CuNPs@Cu(Ⅱ)-AMTD金属有机凝胶复合材料的合成及其催化性能
2018-03-14孙飞飞封其春周映华1
承 勇 孙飞飞 封其春 周映华1,
(1教育部功能分子固体重点实验室,芜湖 241000)
(2安徽师范大学化学与材料科学学院,芜湖 241000)
0 Introduction
During the last two decades,a substantial body of research has been directed toward the synthesis of metal nanoparticles in efforts to explore their special properties and potential applications[1-3].Among various metal particles,copper nanoparticles (CuNPs)have attracted considerable attention because oftheir catalytic,optical,electrical conducting and antifungal/antibacterial properties[4-5].CuNPs are considered as a viable alternative to noble metal nanoparticles in certain fields such as antibacterialapplication,organic synthesis,and catalytic reaction.However,copper nanoparticles are known to be extremely sensitive to oxygen by forming copper oxide nanoparticles and are also apt to aggregate into large sized aggregation due to their high surface energy and high reactivity,resulting in the deterioration of their unique properties[6-7].Therefore,it is desirable to use a matrix that could bind the copper nanoparticles and protect them from oxidizing environment[8-14].Up to now,many strategies have been reported for the preparation of hybrid polymeric gel materials containing metal nanoparticles[15-18],however,there are rare concerned with polymeric gel copper composites.
Polymeric gel material has been regarded as one of the most promising substrate for stabilization of metallic nanoparticles[19-20].Polymeric gel metal composites are viable catalysts because the loosely bound dynamic fibrous structure expected to enhance easy access to the metal nanoparticles[21-23].The catalytic performance and stability of metal nanoparticles can be enhanced by incorporating functional groups such as-NH2,-CONH,and-SiH[24-26].Synthesis of efficient,robust,and reusable homogeneously dispersed copper nanoparticles supported in polymeric gel with enhanced functionalities is still a major challenge.
In this paper,we describe the successful fabrication of copper nanoparticles within Cu(Ⅱ)-AMTD metal-organicgelmatrix.Thecomposites obtained were characterized by IR,SPR,SEM,TEM,EDX and XPS.It displays highly activity in catalytic 4-nitrophenol and other nitroarenes using NaBH4as a reductant performed in an aqueous solution.Considering the wide-ranging potential applications of a metal-organic gel as a host material for a variety of metal nanoparticals,the approach developed here may lead to new possibilitiesforthe fabrication of nanoparticals/metal-organic gel composites endowed with multiple functionalities.
1 Experimental
1.1 Apparatus and materials
All analytical grade solvents and reagents were used without further purification.The precursor 2-amino-5-mercapto-1,3,4-thiadiazole was prepared according to the published procedures[27].4-nitrophenol(4-NP),2-nitrophenol (2-NP),4-nitroaniline (4-NA)and 8-hydroxy-5-nitroquinoline (8-H-5-NQ) were purchased from Aladdin Chemical Reagent Co.Ltd.,China.Cu (Ac)2,N,N-dimethylformamide (DMF)were purchased from Shanghai Lingfeng Chemical Reagent Co.,Ltd.China.Water was deionized and doubledistilled.
Fourier transform infrared spectra (FT-IR)were taken on a Shimadzu FTIR-8400S spectrometer with a KBrpellettechnique.Ultraviolet-visible (UV-Vis)spectra experiments were performed on a Yuanxi UVVis 8000A spectrophotometer.Field emission scanning electron microscope (FESEM)images were obtained using a Hitachi S-4800 scanning electron microscope operating at an accelerating voltage of 5.0 kV.Energy Dispersive X-ray Spectroscopy (EDX)was taken with a Hitachi S-4800 scanning electron microscope.The transmitting electron microscopy (TEM)images were recorded on a JEOL-2011 transmission electron microscope at an accelerating voltage of 200 kV.X-ray photoelectron spectra (XPS)experiments were performed on a Thermo ESCALAB 250XI multifunctional imaging electron spectrometer. Nitrogen adsorption-desorption were obtained using a Nova 2000E surface analyzer.Pore-size distribution was determined from the adsorption branch ofthe isotherms using the Barett-Joyner-Halenda (BJH)method.
1.2Preparation of CuNPs@Cu(Ⅱ)-AMTD composites
DMF solution (1 mL)of AMTD (0.013 4 g,0.1 mmol)was placed in a centrifuge tube.To this,1 mL aqueous solution of Cu(Ac)2(0.020 1 g,0.1 mmol)was added.This immediately results in a dark blue color solution.After little shaking,the mixture was left to stand undisturbed.About 5 minutes,a dark green opacity gel appeared of which the gel state primarily confirmed by the retardation of flow of the materials upon “inversion ofthe centrifuge tube”.The CuNPs@Cu(Ⅱ)-AMTD green powder was obtained by drying the gel at 80℃in the drying oven about 48 h to constant weight(Scheme 1).
1.3 Catalytic reduction of 4-NP and other nitroarenes
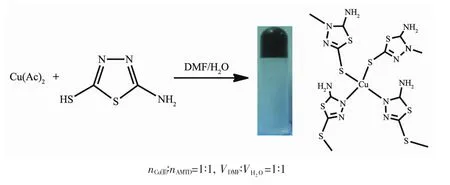
Scheme 1 Formation of Cu(Ⅱ)-AMTD metal-organic gel
To investigate the catalytic efficiency and reusability of the CuNPs@Cu(Ⅱ)-AMTD composites,reduction of 4-NP was performed according to the published procedure[28-29].100 mg CuNPs@Cu(Ⅱ)-AMTD wasadded in a solution containing 50 mL of deionized water to obtain a suspension solution by ultrasonic dispersion.A freshly prepared aqueous solution of NaBH4(56.0 mg,25 mL)was mixed with a 4-NP aqueous solution (10.43 mg,25 mL) (nNaBH4/nsubstrate=5.4)leading to a color change from light yellow to yellow-green.Subsequently,the catalyst suspension solution was added to the mixture under continuous stirring to initiate the reduction reaction.At each time interval,1 mL of the aqueous solution was withdrawn and diluted to 3.0 mL to analyze the reduction efficiency.Since the absorbance of 4-NP is proportional to its concentration in the solution,the ratio of absorbance at time t (At)to that at t=0 (A0)should be equal to the concentration ratio Ct/C0of 4-NP.Consequently,the conversion progress could be directly reflected by the absorption intensity.Therefore,a UVVis spectrophotometer was employed to monitor the progress of the conversion of 4-NP to 4-AP at ambient temperature.For comparison,the control experiment was also carried out under the same experimental condition using AMTD as catalyst.The catalytic activity of the as-prepared CuNPs@Cu(Ⅱ)-AMTD for the reduction of other nitroarenes were also investigated under the same condition.
To test the recyclability of the catalyst,five successive cycles of catalytic reduction were carried out employing a definite amount of catalyst.In the successivecycles,the catalystwascollected by centrifugation from the solution and washed with ethanol and water several times,and used for the next cycling.
2 Results and discussion
2.1Formation of CuNPs@Cu(Ⅱ)-AMTD composites
Cu(Ⅱ)-AMTD metal-organic gel was prepared by copper acetate and AMTD in DMF/H2O as shown in Scheme 1.To understand the coordination behavior between AMTD and Cu2+,we attempted to obtain crystal structure of the related complex,but not successful.In the absence of a suitable single crystal to undertake X-ray crystallography,we proposed that in the gel the nitrogen atom of thiadiazole ring and sulfur atom of thiol are coordinated with two Cu2+,respectively,forming a coordination polymer.Every Cu2+ion is coordinated with four AMTD,two of them act as N donor while the other two act as S donor ligand.Cu2+is linked by AMTD ligand,forming an extended 2D layer network structure.It is noteworthy to point out that there are hydrogen bonds between the solvent H2O and the amino group in the Cu(Ⅱ)-AMTD coordination polymer which formed the metalorganic gel.Considering the reducibility of-NH2in AMTD,we speculate that Cu(Ⅱ)ion would be reduced to copper (0)and CuNPs would existed in the gel matrix.The S atom of thiadiazole ring could also stabilize the CuNPs.Consequently,the Cu(Ⅱ)-AMTD coordination polymer could be served as a directing medium for the synthesis of CuNPs,which were embedded in the gelmatrix,providing a gelcomposites.
2.2 Characterization of CuNPs@Cu(Ⅱ)-AMTD composites
To investigate the morphologies of the composites,SEM were carried out on the xerogel of CuNPs@Cu(Ⅱ)-AMTD.As shown in Fig.1a,the SEM image clearly displays 2D layer fibrillar network,which consistent with the structure of Cu(Ⅱ)-AMTD coordination polymer that we proposed in Scheme 1.
Nitrogen adsorption-desorption measurements were performed to validate the inner architectures of the 2D layernetwork.The nitrogen adsorptiondesorption isotherms and the pore size distribution curve are shown in Fig.2b (inset).The BET surface area of the gel was calculated as about 4 m2·g-1.In addition,the isotherm exhibits a hysteresis loop in the p/p0range of 0.64 to 0.98.This clearly indicates that the gel exhibits a large structural porosity.The pore size distribution of the gel shows a narrow peak in pore size region of 12.4~42.8 nm.That is mainly caused by the accumulation of the gel fibrillar network.
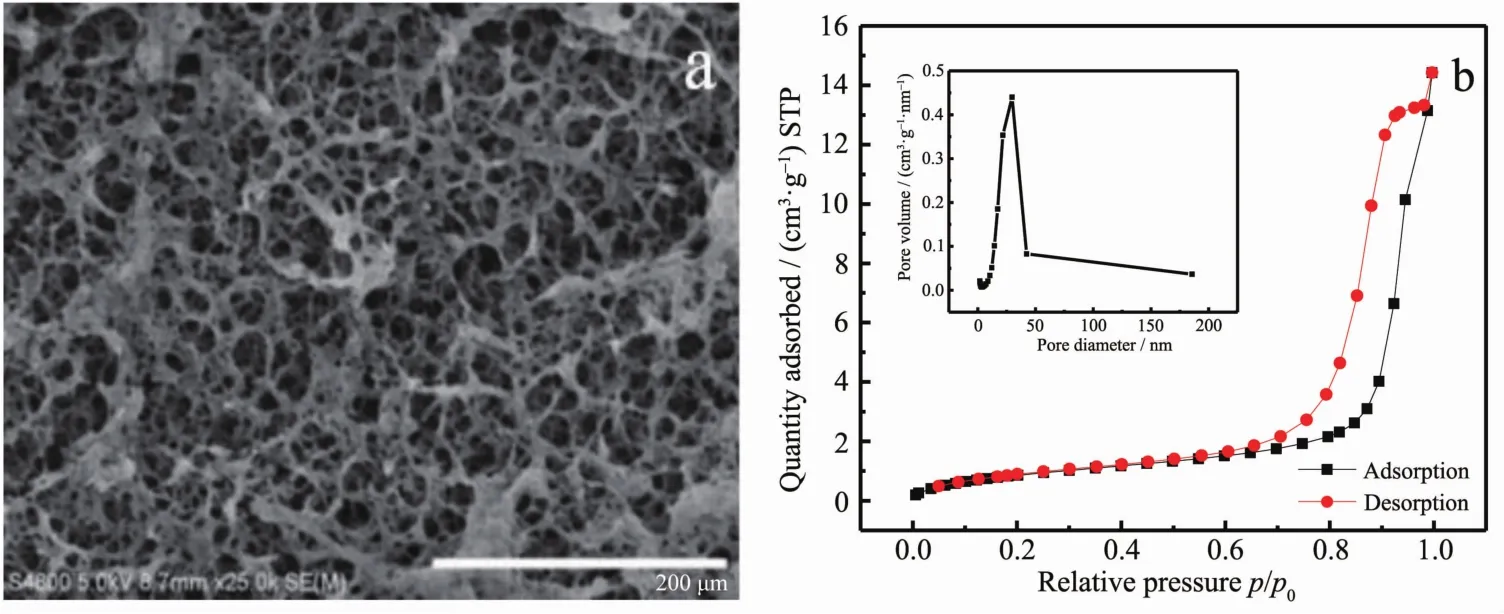
Fig.1 (a)SEM image of CuNPs@Cu(Ⅱ)-AMTD composites;(b)Nitrogen adsorption-desorption isotherms of CuNPs@Cu(Ⅱ)-AMTD composites with corresponding pore-size distribution (inset)calculated by BJH method from the desorption branch
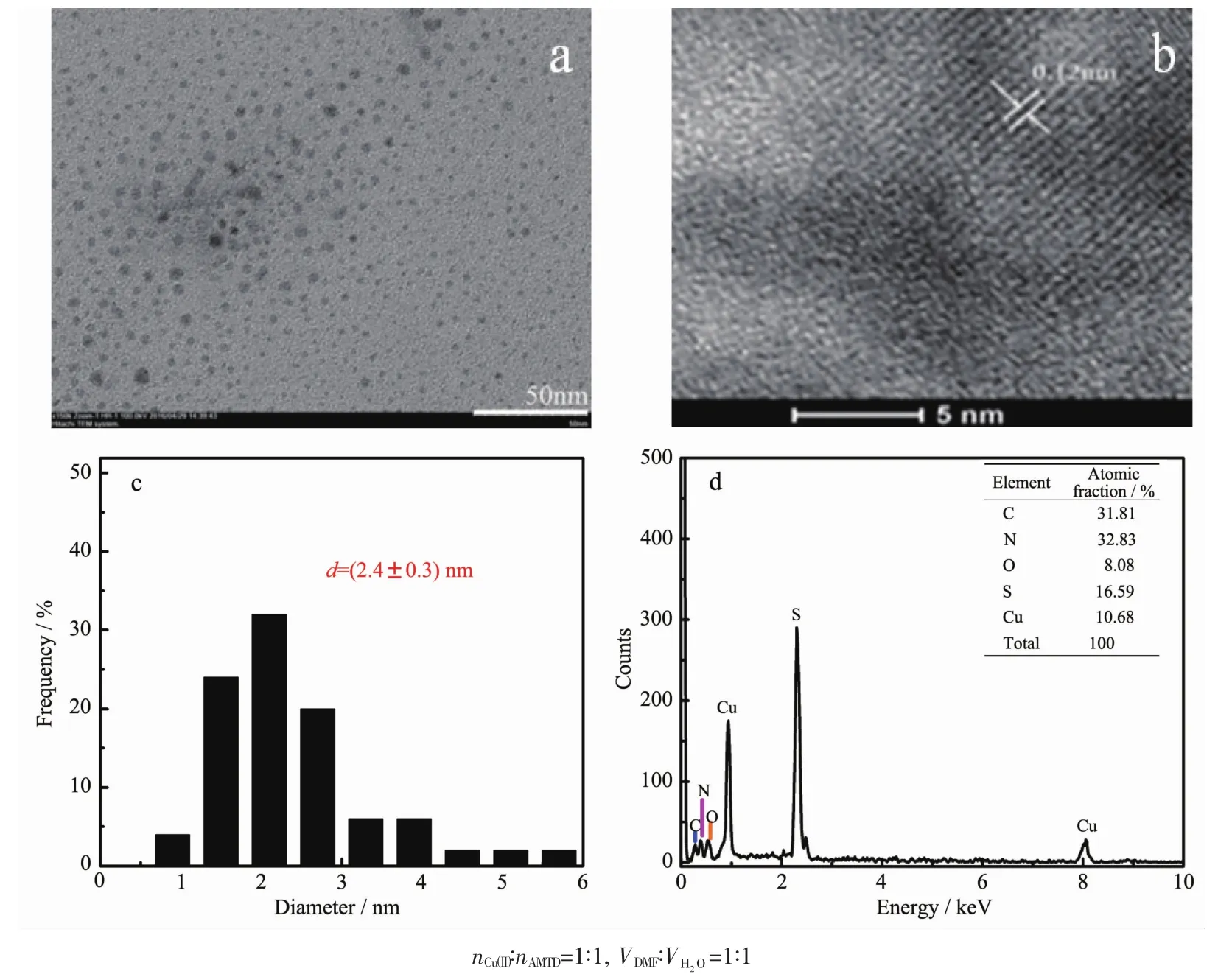
Fig.2 (a,b)TEM images of the CuNPs@Cu(Ⅱ)-AMTD composites;(c)Particles size distribution of CuNPs;(d)EDX image of CuNPs@Cu(Ⅱ)-AMTD composites
TEM measurements were carried out to characterize the morphology and size distribution of copper nanoparticles embedded in the Cu(Ⅱ)-AMTD metal-organic gel matrix.Observing the formed CuNPs directly in the gel is difficult because the gel is too chick to be opaque for the electron beam.In this work,the gel was diluted as a suspension solution for the measurement of TEM.It can be seen that CuNPs were spherical in nature (Fig.2a).The TEM image enlarged version clearly showing the lattice spacing of 0.12 nm,corresponding to the Cu (220)plane (Fig.2b).The particles size distribution shows that a significant amount of nanoparticles are below 5 nm (Fig.2c).Notably,no reflections assignable to metallic CuNPs were present in the XRD pattern of CuNPs@Cu(Ⅱ)-AMTD,possible because the CuNPs content was below the detection limit and/or due to the poor crystallinity of the CuNPs in the composites.Energydispersive X-ray spectroscopy (EDX)was performed on the xerogelwhich determined the elemental composition of copper,chlorine,nitrogen,and carbon(Fig.2d).
To examine the CuNPs in the gel,the samples with different ratios (nCu(Ⅱ)/nAMTD=0.15,0.20,0.25,0.30,0.35)were dissolved in H2O.A small hump at around 345 nm was observed in the UV-Vis absorption spectrum (Fig.3a).It can be assigned to a typical surface plasmon resonance (SPR)excitation from the CuNPs.Furthermore,no apparent SPR absorption band appearing at around 560~600 nm was observed,indicating the absence of large CuNPs[30-32].
Fourier transformed infrared spectroscopy was performed to identify the nature of participation of functional groups present in the ligand and in the corresponding CuNPs@Cu(Ⅱ)-AMTD composites (Fig.3b).The free ligand shows absorption peaks at 3 342,3 254 and 1 609 cm-1that can be assigned to N-H stretching and bending vibrations of-NH2group,which are weaken in the composites.Another broad area was observed near 3 430 cm-1,which could be assigned to the hydrogen bonding of a-OH group of solvent H2O.The peaks at 3 115 and 2 910 cm-1in free ligand assigned to the N-H of thiadiazole ring[33]in the resonance structure are disappeared in the composites,which indicate the coordination of the metal to the N-atom.The weak vibration frequency for-SH stretch at 2 750 cm-1in the ligand is absent in the composites.These observations indicate that the coordination of copper ions with the S atom of thiol group and the N atom of thiadiazole ring in the gel.
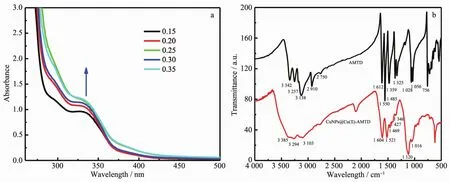
Fig.3 (a)UV-Vis absorption spectra of CuNPs in the gel matrix with different ratios (nCu(Ⅱ)∶nAMTD=0.15,0.20,0.25,0.30,0.35);(b)FT-IR spectra obtained from AMTD and CuNPs@Cu(II)-AMTD composites
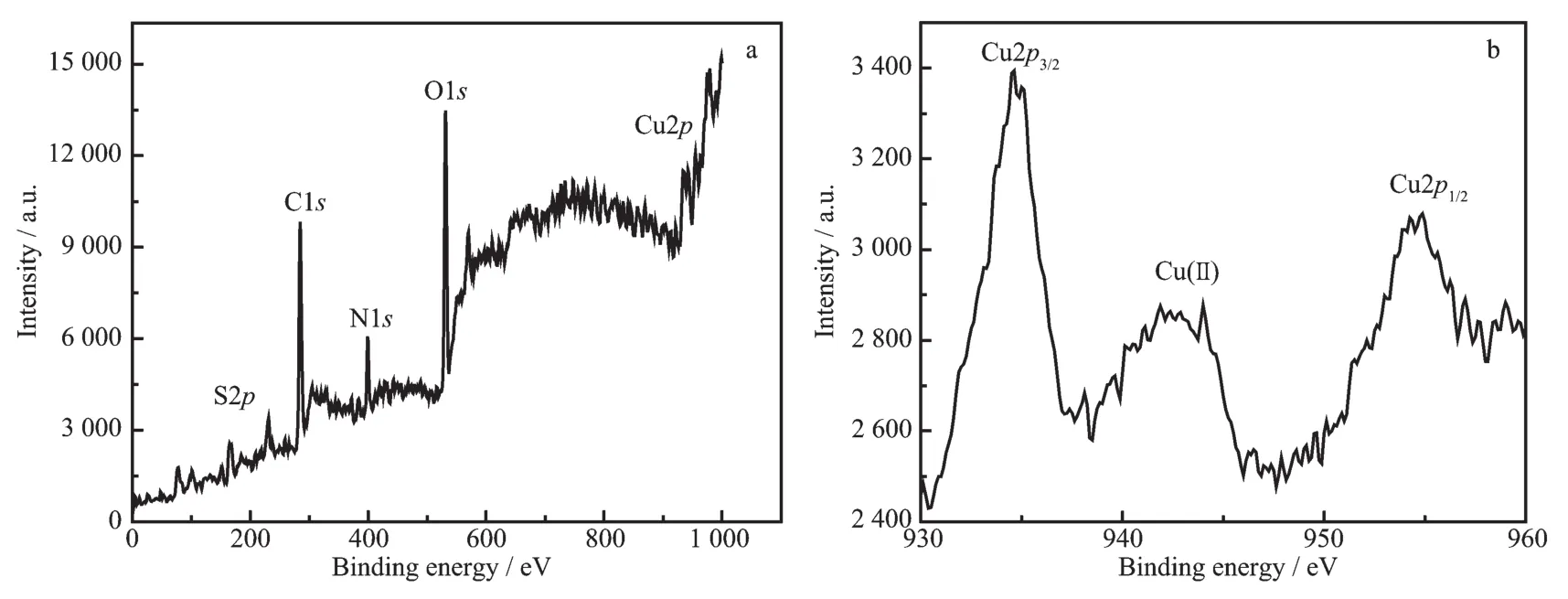
Fig.4 (a)XPS of CuNPs@Cu(Ⅱ)-AMTD composites;(b)High-solution XPS of Cu2p electrons
For the purpose of gaining insight into components of the CuNPs@Cu(Ⅱ)-AMTD composites,XPS survey spectra were performed.As shown in Fig.4a,five major peaks of S2p,C1s,N1s,O1s,Cu2p obviously emerged in the spectrum,indicating that CuNPs@Cu(Ⅱ)-AMTD prepared here were mainly composed of C,O,N,S and Cu.The oxidation state of copper in samples was also studied by XPS.As shown in Fig.4b,two fitting peaks at 934.6 and 954.3 eV were observed in the Cu2p XPS spectrum,corresponding to the binding energies of Cu2p3/2and 2p1/2,respectively,indicating the existence of Cu(0)or Cuガin the composites[34-35].Furthermore,there was a peak displayed around 942.7 eV, demonstrating the existence of Cu(Ⅱ)[36],which maybe attribute to Cu(Ac)2-AMTD coordination polymerin the composites(Scheme 1).
2.3 Catalytic reduction of nitroarene
4-Nitrophenol (4-NP) is one ofthe most hazardous and toxic organic pollutants in waste-water generated from agricultural and industrial sources.4-Aminophenol(4-AP)is also an important intermediate on the manufacture of antipyretic and analgesic drugs.The development of an effective catalysts is expected for the reduction of 4-NP to 4-AP[37-40].So,we choose the reduction of 4-NP as a model reaction to evaluate the catalytic activity of our synthesized composites.
Normally,the 4-NP solution shows an absorbance peak at 317 nm under neutral conditions,which shifts to 400 nm after adding NaBH4because of the formation of 4-nitrophenolate ions via deprotonation(pKa=7.2)[42-43].During the reduction of 4-NP to 4-AP,the intensity of the absorption peak at 400 nm gradually decreased because of the consumption of 4-NP,resulting in the fading and ultimate bleaching of the yellow-green color of 4-nitrophenolate.Meanwhile,the generation of reduction product 4-AP led to a new UV-Vis peak at approximately 300 nm (Fig.5a)[44].
Fig.5b presents the time-dependent evolution of theUV-Vis spectra of this reaction with CuNPs@Cu(Ⅱ)-AMTD as catalyst,showing a successive intensity decrease in the absorption peak at 400 nm,along with a concomitant appearance of a new peak at about 300 nm.All the spectra intersect each other at two points,indicating that the nitro compound was gradually converted to 4-AP without the formation of byproducts[45].After a 12 min reaction,the peak at 400 nm ascribed to 4-nitrophenolate disappeared,indicating the complete transformation of the 4-NP.Meanwhile,at the end of the reaction,the peak for 4-NP almost disappeared and only the peak for 4-AP could be observed,thus suggesting the presence of product with high purity.Additionally,the control experiment was performed by taking AMTD instead of CuNPs@Cu(Ⅱ)-AMTD.In this case,the intensity of the peak at 400 nm remained unchanged even after 12 h,confirming the catalytic role of CuNPs on the reduction reaction.
Given that the concentration of NaBH4significantly exceeds that of 4-NP in the reaction system,the reduction rate was roughly independent of NaBH4concentration.Generally,the kinetics can be considered as pseudo-first-order with respect to 4-NP[46].In this case,the consumption of 4-NP is given by

where rtis the consumption rate of 4-NP at time t,Ctis the concentration of 4-NP at time t,and k is the first-order rate constant.
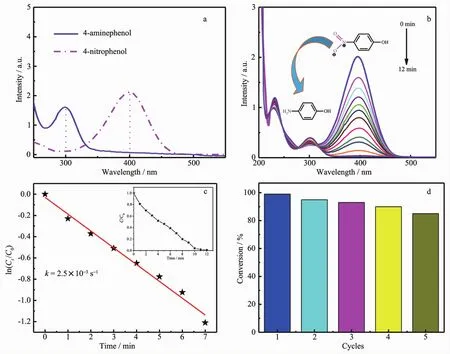
Fig.5 (a)UV-Vis absorption spectra of 4-nitrophenol and 4-aminephenol;(b)Successive UV-Vis absorption spectra of the reduction of 4-nitrophenol by NaBH4in the presence of CuNPs@Cu(Ⅱ)-AMTD composites;(c)Plot of ln(Ct/C0)against time (inset:Ct/C0~t);(d)Conversion efficiency of 4-NP in five successive cycles
Fig.5c shows ln(Ct/C0)versus reaction time for the reduction of 4-NP using the CuNPs@Cu(Ⅱ)-AMTD as catalyst.ln(Ct/C0) was obtained from the relative intensity of the absorption at 400 nm because the absorption intensity of 4-NP is proportional to its concentration in the medium.The linear relationship between ln(Ct/C0)and reaction time (t)confirms the pseudo-first-order kinetics.The rate constant (k)of the catalytic reaction was 2.5×10-3s-1from the slope of the linear plot,which is higher than that of CuNPs(1.6×10-3s-1)[47],but was lower than that of Cu-TOCNF(2.4×10-2s-1)[48]and CuNCs (8.2×10-3s-1)[49].The ratio of the rate constant to the catalyst weight was 0.025 s-1·g-1.Generally,the rate constant of catalytic reaction is affected by the concentration or loading amount of CuNPs.
Stability and recyclability is of great importance for the practical applications of catalysts.Recycling andreuseofCuNPs@Cu(Ⅱ)-AMTD werefurther examined under the same reaction conditions as that of the first cycle.As shown in Fig.5d,the catalyst can be successfully recycled and reused for five successive cycles of reaction with a conversion efficiency (~82%),indicating the stable and high recycling efficiency of the CuNPs@Cu(Ⅱ)-AMTD.The good recyclability of CuNPs@Cu(Ⅱ)-AMTD should be attributed to the strong stabilization ability of metal-organic gel matrix toward the CuNPs.These results clearly demonstrate that the Cu(Ⅱ)-AMTD metal-organic gel are excellent supporting carrier for CuNPs growth and immobilization because of their high specific surface area and interwoven fibrous structure properties.
The CuNPs@Cu(Ⅱ)-AMTD can also be used for the reduction of other nitrobenzene analogues such as 4-NA,2-NP and 8-H-5-NQ.Here,we choose to run the reactions in the presence of CuNPs@Cu(Ⅱ)-AMTD catalyst and nitroarene with NaBH4to clearly monitor the conversion efficiency of the reaction.As shown in Fig.6,the composite exhibits high reactivity with excellent yields toward these nitroarenes compounds.It is also interesting to note that the 2-nitrophenol displays better conversion efficiency than other analogues.
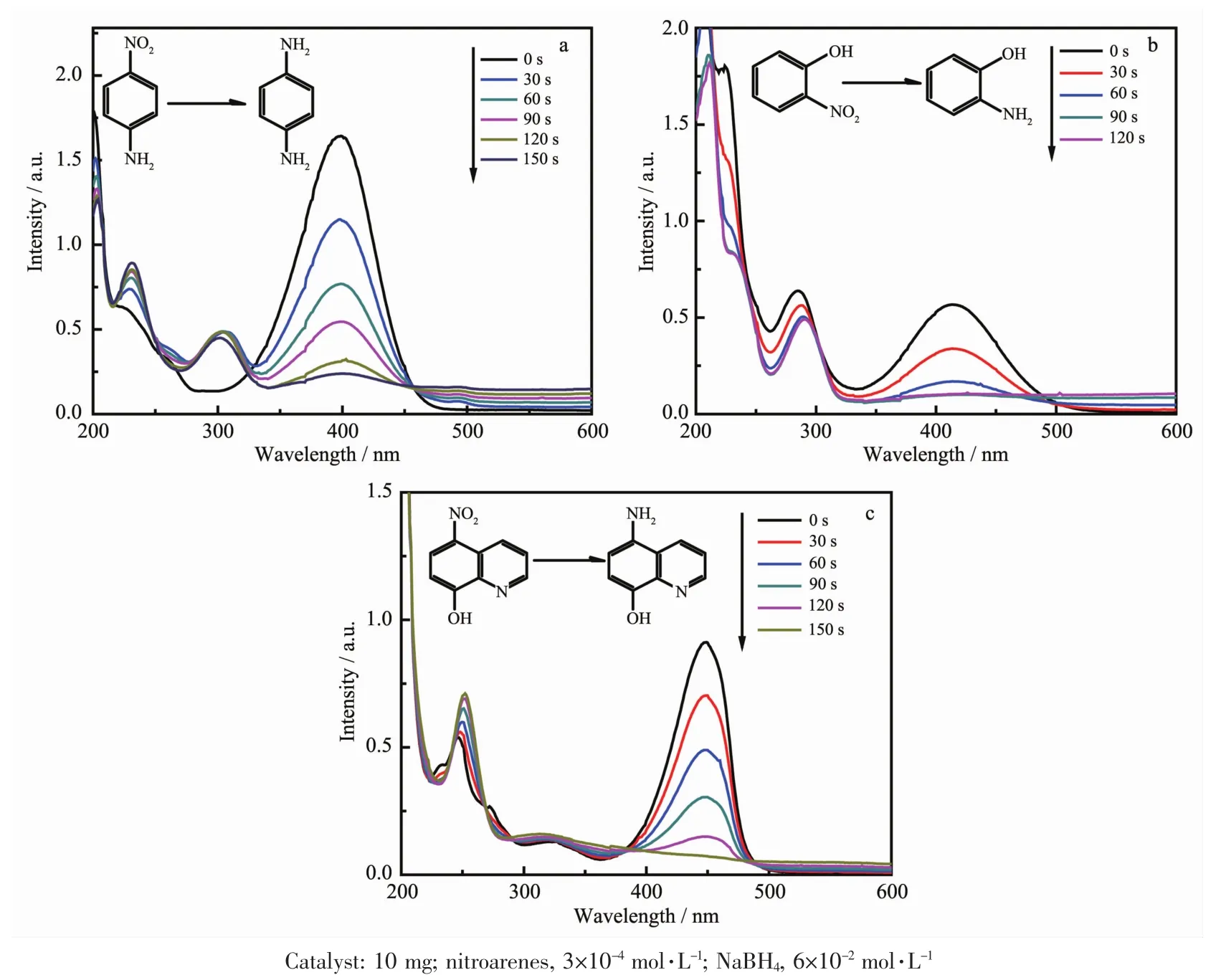
Fig.6 Reduction of various nitroarenes using CuNPs@Cu(Ⅱ)-AMTD as catalyst:(a)4-NA,(b)2-NP and (c)8-H-5-NQ
The mechanism of the catalytic reaction could be explained by the Langmuir-Hinshelwood mechanism(Fig.7).NaBH4ionized in water to offer BH4-,providing surface hydrogen for the reaction.BH4-acted as the electron donor,whereas 4-NP acted as the electron acceptor.CuNPs act as an electronic relay agent to overcome the kinetic barrier,allowing the electron transfer from BH4-to 4-NP[43].Because of the strong adsorbing ability of the Cu(Ⅱ)-AMTD metal-organic gel,NaBH4and 4-NP could be rapidly adsorbed on the surface of gel,where the copper particles could relay electrons from the donor of BH4-to the acceptor of 4-NP,and promote the occurrence of reduction reaction.Therefore,the high catalytic activity arises from the synergistic effect of Cu(Ⅱ)-AMTD metalorganic gel and CuNPs:the high adsorption and electron transfer ability.
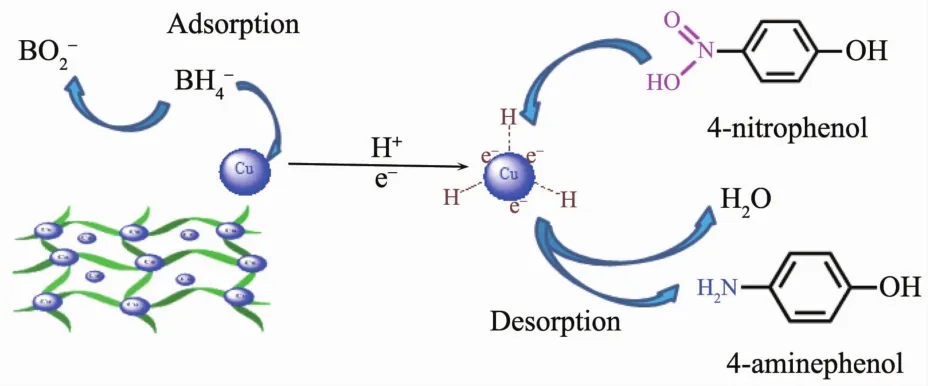
Fig.7 Proposed the mechanism of the reduction of 4-NP to 4-AP
3 Conclusions
In conclusion,a novel composites CuNPs@Cu(Ⅱ)-AMTD were obtained by using Cu(Ⅱ)-AMTD metalorganic gel as a platform for in situ growth copper nanoparticleswithin gelmatrix.The as-prepared material is an efficient catalyst in the reduction of nitroarenes compounds.Such composites were thus expected to have the potential to be a new class of highly efficient,fully renewable heterogeneous catalyst for industrial applications.
[1]ScholtenJD,LealBC,DupontJ.ACSCatal.,2012,2:184-200
[2]Iablokov V,Beaumont S K,Alayoglu S,et al.Nano Lett.,2012,12:3091-3096
[3]David C,De Abajo F J G.J.Phys.Chem.C,2011,115:19470-19475
[4]Villanueva M E,Diez A M R,Gonzalez J A,et al.ACS Appl.Mater.Interfaces,2016,8:16280-16288
[5]Manthiram K,Beberwyck B J,Alivisatos A P.J.Am.Chem.Soc.,2014,136:13319-13325
[6]Park B K,Jeong S,Kim D,et al.J.Colloid Interface Sci.,2007,311:417-424
[7]BenaventeE,LozanoH,Gonzalez G.Recent Pat.Nanotechnol.,2013,7:108-132
[8]Kanninen P,Johans C,Merta J,et al.J.Colloid Interface Sci.,2008,318:88-95
[9]Ruiz P,Munoz M,Macanás J,et al.Chem.Mater.,2010,22:6616-6623
[10]Mallick S,Sharma S,Banerjee M,et al.ACS Appl.Mater.Interfaces,2012,4:1313-1323
[11]Bogdanovi U,Vodnik V,Mitri M,et al.ACS Appl.Mater.Interfaces,2015,7:1955-1966
[12]Gholinejad M,Jeddi N.ACS Sustainable Chem.Eng.,2014,2:2658-2665
[13]Tokarek K,Hueso J L,Kustrowski P,et al.Eur.J.Inorg.Chem.,2013:4940-4947
[14]Li B J,Li Y Y,Wu Y H,et al.Mater.Sci.Eng.C,2014,35:205-211
[15]Che Y,Zinchenko A,Murata S.J.Colloid Interface Sci.,2015,445:364-370
[16]Das D,Kar T,Das P K.Soft Matter,2012,8:2348-2356
[17]Maity I,Rasale D B,Das A K.Soft Matter,2012,8:5301-5308
[18]Roy S,Banerjee A.Soft Matter,2011,7:5300-5308
[19]SHENG Li-Ying(沈利英),YU Hai-Tao(于海涛),HE Xuan(何璇),et al.Chin.J.Org.Chem.(有 机化学),2009,29(4):548-563
[20]WU Ting(吴 婷 ).Thesis for the Master of Anhui Normal University(安徽师范大学硕士论文),2014.
[21]Lu Y,Spyra P,Mei Y,et al.Macromol.Chem.Phys.,2007,208:254-261
[22]Otari S V,Patil R M,Waghmare S R.Dalton Trans.,2013,42:9966-9975
[23]Díaz D D,Kuhbeck D,Koopmans R J.Chem.Soc.Rev.,2011,40:427-448
[24]Li J,Zhu J W,Liu X H.Dalton Trans.,2014,43:132-137
[25]Zheng Y,Wang A Q.J.Mater.Chem.,2012,22:16552-16559
[26]Gupta N R,Prasad B LV,Gopinath G S,et al.RSC Adv.,2014,4:10261-10268
[27]Misra U,Shukla S,Gurtu S,et al.Boll.Chim.Farm.,1995,134:492-496
[28]Liang M,Su R,Huang R,et al.ACS Appl.Mater.Interfaces,2014,6:4638-4649
[29]Liang M,Wang L,Liu X,et al.Nanotechnology,2013,24:245601
[30]Lisiecki I,Piled M P.J.Phys.Chem.,1995,99:5077-5082
[31]Salzemann C,Lisiecki I,Brioude A,et al.J.Phys.Chem.B,2004,108:13242-13248
[32]Mott D,Galkowski J,Wang L Y,et al.Langmuir,2007,23:5740-5745
[33]Chufán E E,Pedregosa J C,Borrás J.Vib.Spectrosc.,1997,15:191-199
[34]Bradwell D J,Osswald S,Wei W F,et al.J.Am.Chem.Soc.,2011,133:19971-19975
[35]Balogh L,Tomalia D A.J.Am.Chem.Soc.,1998,120:7355-7356
[36]Wu C K,Yin M,OBrien S,et al.Chem.Mater.,2006,18:6054-6058
[37]AdityaT,PalbA,PalT.Chem.Commun.,2015,51:9410-9431
[38]Zhao P X,Feng X W,Huanga D S,et al.Coord.Chem.Rev.,2015,287:114-136
[39]Wang C,Cigand R,Salmon L,et al.Angew.Chem.Int.Ed.,2016,55:3091-3095
[40]Wang C,Salmon L,Li Q,et al.Inorg.Chem.,2016,55:6776-6780
[41]JIANG Jun (姜俊),LI Gang (李钢),KONG Ling-Hao (孔令浩).Acta Phys.-Chim.Sin.(物理化学学报),2015,31(1):137-144
[42]Yang M Q,Weng B,Xu Y J.Langmuir,2013,29:10549-10558
[43]Wang H,Dong Z X,Na C Z.ACS Sustainable Chem.Eng.,2013,1:746-752
[44]Liu C H,Chen X Q,Hu Y F,et al.ACS Appl.Mater.Interfaces,2013,5:5072-5079
[45]Liang M,Wang L,Su R X,et al.Catal.Sci.Technol.,2013,3:1910-1914
[46]Zhu C H,Hai Z B,Cui C H.Small,2012,8:930-936
[47]Deka P,Deka R C,Bharali P.New J.Chem.,2014,38:1789-1793
[48]Bendi R,Imae T.RSC Adv.,2013,3:16279-16282
[49]Zhang P H,Sui Y M,Xiao G J,et al.J.Mater.Chem.A,2013,1:1632-1638
