Calcium channel α2δ1 subunit as a novel biomarker for diagnosis of hepatocellular carcinoma
2018-03-08SanaAmhimmidBadrMaryanWaheebFahmiManalMahmoudNomirMamdouhMohammadElShishtawyBiochemistryDepartmentFacultyofPharmacyMedicalOncologyUnitInternalMedicineDepartmentFacultyofMedicineClinicalPathologyDepartmentStudentsHospitalMans
Sana Amhimmid Badr, Maryan Waheeb Fahmi, Manal Mahmoud Nomir, Mamdouh Mohammad El-ShishtawyBiochemistry Department, Faculty of Pharmacy; Medical Oncology Unit, Internal Medicine Department, Faculty of Medicine; Clinical Pathology Department, Students Hospital, Mansoura University, Mansoura 556, Egypt
Introduction
Liver cancer is considered to be a global health dilemma.Globally, it is the fifth most prevalent malignancy and the second leading cause of cancer related mortality1. In Egypt,liver cancer is the most common malignancy in men and the second most common cancer in women, as declared by the National Population-Based Registry Program of Egypt 2008–20112. Hepatocarcinogenesis is considered a complex,slow, and progressive multistep process3.
Huge efforts have been undertaken to achieve the early detection or prevention of this fatal cancer in an attempt to improve patient survival and quality of life. The identification of new accurate, valid and non-invasive serum proteins as diagnostic biomarkers for hepatocellular carcinoma (HCC) forms part of HCC research4.
Annexin A2 (ANXA2, annexin II), also known as placental anticoagulant protein IV and p36, is a member of the calcium and phospholipid-binding protein families. The protein,which is expressed in endothelial cells, macrophages,mononuclear cells, and several cancers exists as a monomer or heterotetramer5. The main biochemical characteristics of annexin A2 are the calcium-dependent association with phospholipids and an actin cytoskeleton. It is involved in endocytosis, exocytosis and cell polarization6, and is considered a cellular redox regulatory protein7, and interacts with tissue plasminogen activator and its substrate,plasminogen8. The aberrant expression of annexin A2 was found in several solid cancers, such as HCC9, lung10, breast11,gastric12, and colorectal cancer13. The overexpression of annexin A2 was also detected in hematological malignancies such as acute lymphoblastic leukemia14and acute promyelocytic leukemia15. It regulates several steps in the carcinogenesis process, such as tumor cell to cell adhesion,growth, invasion, metastasis, and angiogenesis16. Annexin A2 prevents radiation-induced apoptosis17, and regulates immune responses18.
Voltage-gated calcium channels (VGCC) exist throughout the body and perform several key physiological functions.VGCC activation is mandatory for the release of neurotransmitters at synapses in the brain in addition to spinal and hormone secretion19. The α2/δ subunit is a transmembrane protein that forms glycosyl-phosphatidylinositol(GPI) anchored protein20, which can change the calcium channel function by increasing the rate and voltage dependent calcium channel gating21. The VGCC α2/δ subunit is a member of the α2/δ subunit family, which is encoded by CACNA2D1 and has five isoforms. The α2/δ subunit is as a functional hepatic cancer stem cell, a marker of tumor-initiating cells and controls calcium influx into liver tumor-initiating cells through L and N-type voltage-gated calcium channels. The overexpression of α2/δ was attributed to the increase of Ca2+in Hep-11 cells, whereas the knockdown of α2/δ in Hep-12 cells contributed to a decrease of Ca2+. The α2/δ subunit upregulated the expression of the a1B, a1C, and a1F subunits. Moreover, α2/δ knockdown inhibited ERK1/2 phosphorylation and enhanced apoptosis22,furthermore, is implicated in extracellular signaling23.
This work aimed to evaluate serum annexin A2 and voltage-gated calcium channel subunit α2/δ1 as potential new diagnostic biomarkers for HCC.
Patients and methods
Patients
This study is considered to represent a case-control study,which was conducted between February and October in 2016.Serum blood samples were collected and analyzed from 75 patients and from 15 apparently healthy volunteers who were classified into three groups.
Group I: 50 patients with HCC, who presented and were treated at the Oncology Center of Mansoura University,Mansoura, Egypt.
Group II: 25 patients with chronic hepatitis C infection and cirrhosis without any evidence of HCC, who were treated at the Outpatient Clinics of Specialized Medical Hospital,Mansoura University, Mansoura, Egypt.
Group III: 15 apparently healthy volunteers comprise the normal control group; all were without histories of any medical disorder, acute or chronic liver diseases, and were sero-negative for hepatitis B surface antigen (HBsAg),hepatitis C virus antibodies (anti-HCV), and human immunodeficiency virus (HIV) antibodies.
1.2.2 抗H7N9病毒治疗 (1)奥司他韦胶囊(意大利Roche S.p.A公司)150 mg口服,每日两次;(2)在取得患者和家属同意并签署“知情同意书”后实施奥司他韦雾化治疗,将奥司他韦75 mg(1粒)捣碎后转入雾化器杯里,加生理盐水7 mL,并充分搅拌后雾化(flexicare口含型雾化器,富利凯医疗用品有限公司),每日雾化两次。
The protocol of the study was reviewed and approved by the Research Ethics Committee of the Faculty of Pharmacy,Mansoura University, Mansoura, Egypt. The study complied with the ethics principles of the 1964 declaration of Helsinki and all subsequent revisions in addition to the guidelines of Good Clinical Practice (GCP).
All participants in the study have signed informed consent.
Methods
All patients and the volunteers in the control group were subjected to full a clinical evaluation (clinical examination history collection). The laboratory investigations included complete blood count (CBC), liver function tests, including alanine aminotransferase (ALT), aspartate aminotransferase(AST), gamma glutamyl transferase (GGT), prothrombin time (PT), international normalized ratio (INR), serum albumin, serum total bilirubin (T. Bil), viral markers (HCV-antibodies, HBs-antigen, and HIV-antibodies), serum creatinine, random blood sugar, and serum levels of alphafetoprotein (AFP), annexin A2, and the α2δ1 subunit. The radiological investigations included ultrasonography for all patients (group I, II) and the control group. Ultrasound studies were conducted to evaluate the liver portal vein and spleen. Ultrasonography was used for the initial detection of hepatic focal lesions, and triphasic computed tomography(CT) scans or dynamic contrast-enhanced magnetic resonance imaging (MRI) was used to confirm the diagnosis of HCC in the presence of underlying cirrhosis. The diagnosis of HCC was in accordance with the National Comprehensive Cancer Network guidelines version 1.201624.All patients in the enrolled groups were > 18 years old,performance status (PS) ≤ 2 according to Eastern Cooperative Oncology Group, performance status in addition to Child–Pugh classification A. Patients with other types of malignancy, pregnancy, advanced organ failure,active infection in addition to advanced medical comorbidity were excluded from the study.
Blood sample collection and storage
All blood samples were collected at time of diagnosis. Fasting blood samples (5 mL) were collected from all patients and control groups and divided into two portions. The first portion was collected within tubes containing ethylenediaminetetra-acetic acid (EDTA) and analyzed within 5 h. The second portion was collected in a Monovette without additives. This blood was left to clot for 20–30 min at 37°C, followed by centrifugation at 1700 rpm for 15 min to obtain the clear non-hemolyzed serum. When the analysis was not performed immediately, the samples were frozen and stored at 80°C until use.
Biochemical analysis parameters
Complete blood counts, liver function tests in the form of serum ALT, AST, GGT activities, and total bilirubin and albumin levels, prothrombin time and INR, serum creatinine, and random blood sugar levels were measured by using commercially available kits.
Viral markers (HBsAg, anti-HCV antibodies, and HIV antibodies) and serum AFP level was measured by using ELISA kits (Diametra, Italy).
The serum concentration for annexin A2 was determined by a commercially available ELISA kit (Mybiosource, catalog no: MBS 760680, USA).
Statistical analysis
IBM SPSS Advanced Statistics version 20 (SPSS Inc.,Chicago, IL, USA) was used to compute the statistical analysis. A Chi-square test was used to estimate the relationship between the qualitative variables, which were presented as frequencies and percentages. The quantitative variables were presented as the mean ± standard deviation or median percentile of the inter-quartile range (25thto 75th) for data that were not normally distributed (SD > 25% of mean)and were analyzed by using the Mann-Whitney test;comparison between two / all study groups was conducted by using the Kruskal-Wallis test (non-parametric ANOVA). The receiver operating characteristic (ROC) curve was applied to identify the best cut-off values for annexin A2, the α2δ1 subunit, and AFP. P values of < 0.05 and < 0.01 were considered to represent significant and highly significant changes respectively.
Results
Group I comprised 50 patients with HCC (11 women and 39 men; 50–73 years old; mean age, 60.34 ± 6.19 years old), who had performance status ≤ 2, and Child- Pugh score A at the time of diagnosis. All patients developed HCC in addition to cirrhosis, which was related to chronic HCV infection and all patients were sero-negative for HBV and HIV antibodies.Fifteen patients (30%) had single hepatic focal lesions, 35 patients (70%) had multiple hepatic focal lesions. Sixteen patients (32%) had unilobar tumors, 34 patients (68%) had bilobar tumors, and portal vein thrombi were detected in 22 patients (44%). Twenty-nine patients (58%) had tumors ≤ 5 cm and 21 patients (42%) had tumors > 5 cm. Metastasis was detected in 7 patients (14%).
Group II comprised 25 patients with cirrhosis (13 women and 12 men; 20–78 years old, mean age, 50 ± 13.23 years old). All patients were sero-positive for HCV antibodies and sero-negative for HBV and HIV infections.
Group III comprised 15 apparently healthy volunteers (4 women and 11 men, 20–36 years old, mean age, 26 ± 3.6 years old). All patients were sero-negative for HCV, HBV,and HIV antibodies, with normal clinical; laboratory, and radiological findings.
The patients in group I and group II experienced a significant decrease in the concentration of serum albumin and in platelet count compared with the group III, with a non-significant increase in serum albumin concentration and platelet count in group I compared with group III and a highly significant increase in serum was detected in group I and II in comparison with group III (Table 1).
Patients in group I and group II displayed a significant increase in serum AST, ALT, and GGT activities and total bilirubin concentration compared with group III, whereas no significant difference in serum GGT activity was observed in patients in group I compared with patients in group II(Table 1).
No significant difference was found in the serum levels of annexin A2 or the α2δ1 subunit and the clinicopathological features of patients with HCC with age, sex, tumor size,portal vein invasion, metastasis, Child Pugh score of 5 and 6,and performance status (1 and 2). For AFP, no statistical significance was found, except for tumor size (P = 0.02). The serum level of AFP was higher in larger tumors (Table 2).
Measurement of serum annexin A2 for HCC diagnosis
The serum levels of annexin A2 were significantly higher in group I (mean = 10.04 ng/mL) and group II (mean = 9.31 ng/mL) compared with group III (mean = 0.296 ng/mL) at P< 0.001. However, no significant difference was observed in the annexin A2 serum level between patients in group I and group II (Table 3, Figure 1).
When ROC calculation and analysis was used to study thepotential of annexin A2 as a biomarker for the diagnosis of HCC at a cut-off ≥ 9.82 ng/mL, the area under the curve(AUC) was 0.606 and the P-value was 0.135. These values were not statistically significant for diagnosis and the diagnostic accuracy was 49% (Table 4, Figure 2).
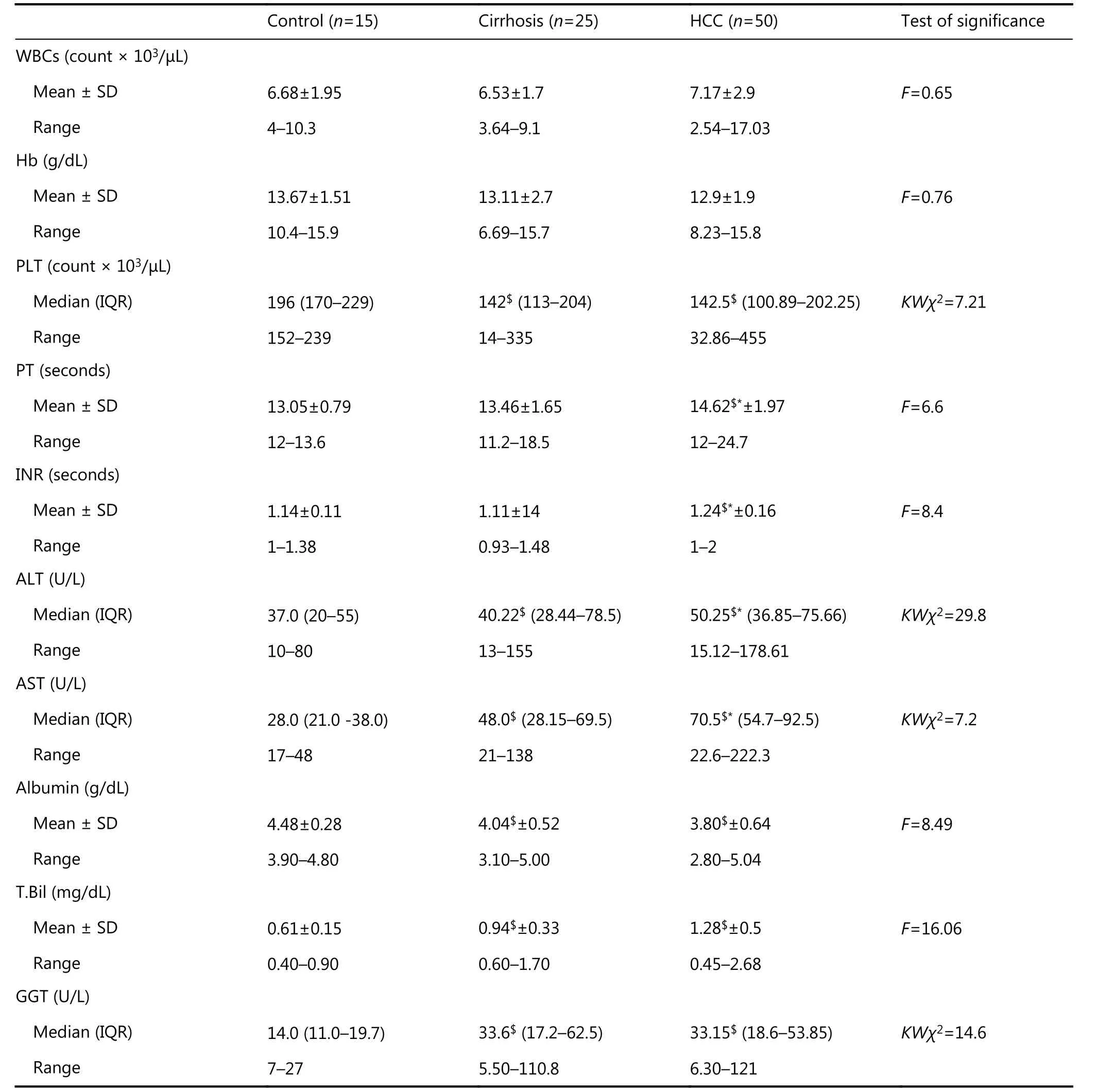
Table 1 Laboratory parameters among groups
Detection of serum α2δ1 subunit for HCC diagnosis
The serum level of the α2δ1 subunit was significantly higher with patients in group I (mean = 20.12 ng/mL) comparedwith the patients in group II (mean = 10.41 ng/mL, P <0.001) or the group I (mean = 10.2 ng/mL, P < 0.001), (Table 3, Figure 1).

Table 2 Comparison among the annexin A2 (ng/mL), α2δ1 subunit (ng/mL) and AFP (ng/mL), as well as clinicopathological features of HCC
When the ROC curve was applied to select the cut-off value, the data revealed that the serum level of the α2δ1 subunit at the cut-off value ≥ 14.22 ng/mL had a sensitivity of 100%, a specificity of 96%, a positive predictive value (PPV)of 98%, and a negative predictive value (NPV) of 100%. The AUC showed a high accuracy for the α2δ1 subunit (AUC =0.97) compared with AFP (AUC = 0.94) or annexin A2 (AUC= 0.606), as illustrated in (Table 4, Figure 2), so the serum level of α2δ1 subunit may be a novel diagnostic biomarker.
Discussion
In majority of patients, HCC has a high prevalence and dismal prognosis. The screening of high risk groups by serum AFP level and hepatic ultrasonography every 6 months is a common practice25. Despite this, AFP is usually not elevated in the early stages of HCC26. The updated American Association for Study of Liver Diseases guidelines no longer recommend the serum AFP level as part of the diagnostic procedure for HCC. AFP does not provide a sensitive or specific test for HCC and is elevated in some cases of intrahepatic cholangiocarcinoma, metastasis to liver, and other non-malignant conditions24,26. This work revealed that an AFP of < 400 ng/mL in approximately 21 of 50 (42%) of the patients with HCC and cirrhosis related to chronic HCV infection. No statistically significant correlation was observed between the serum AFP level clinicopathological features(age, sex, vascular invasion, or metastasis), except for tumor size. The serum AFP at the cut-off level of 418.5 ng/mL had asensitivity of 58%, a negative predictive value of 54%, and a diagnostic accuracy of 72%. These data were in agreement with the analysis performed on AFP levels in 309 pathologically confirmed patients with HCC, which showed that serum AFP was not a sensitive biomarker, especially in the early stages of HCC. This analysis contributed to the inconclusive correlation between AFP and many clinical pathological features in most studies with an arbitrarily chosen AFP cut-off in different populations and in different geographic areas26.
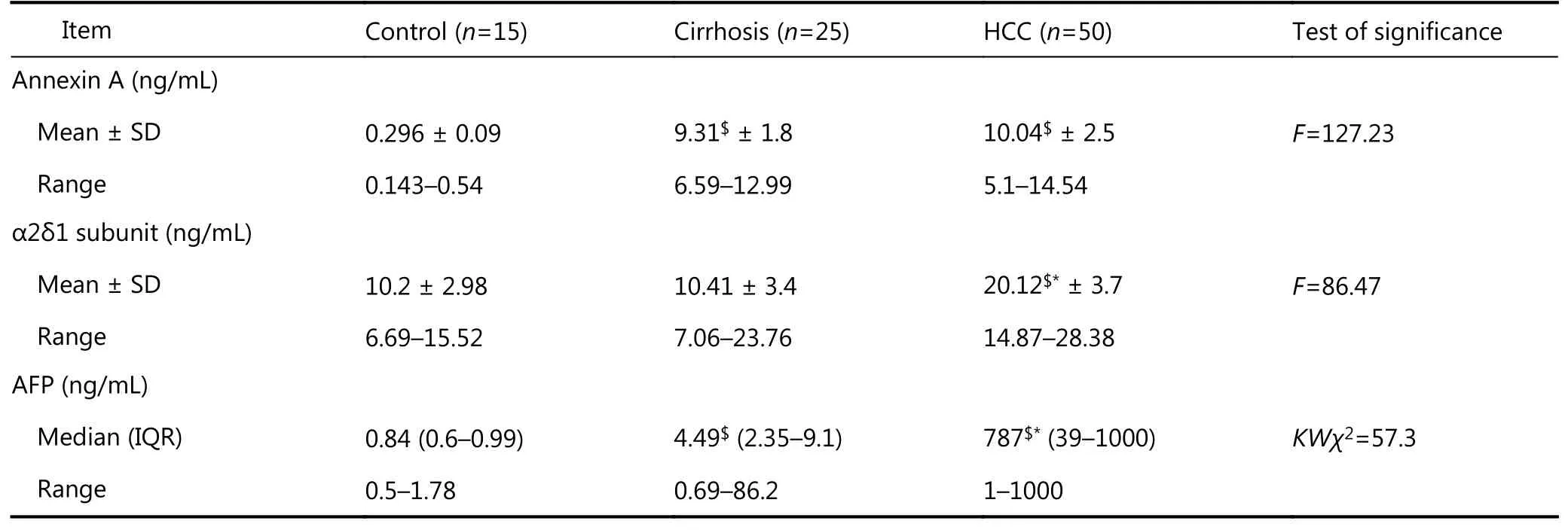
Table 3 Serum levels of annexin A, α2δ1 subunit and AFP in studied groups using ELISA tests
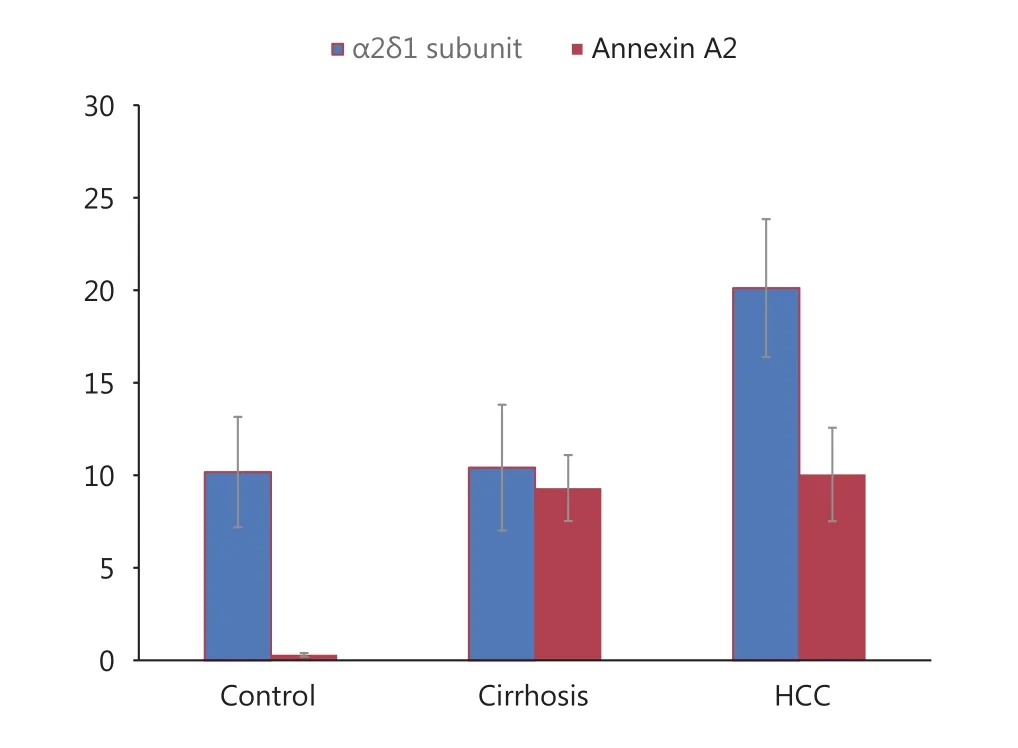
Figure 1 Serum levels of annexin A2 (ng/mL) and α2δ1 subunit(ng/mL)(mean ± SD) in studied groups.
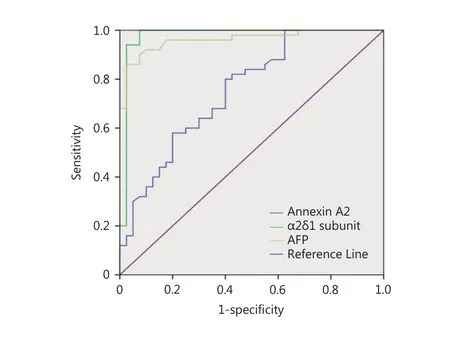
Figure 2 ROC curve comparing annexin A2, α2δ1 subunit and AFP in HCC patients.

Table 4 Diagnostic accuracy of annexin A2, α2δ1 subunit and alpha fetoprotein (AFP) in HCC patients
This study found that the serum levels of annexin A2 were significantly elevated in patients with HCC and patients with cirrhosis compared with the normal control group at P <0.001; but no statistically significant difference was observed in serum annexin A2 levels between patients with HCC and patients with cirrhosis and no statistically significant correlation was found serum annexin A2 level and clinicopathological features, such as age, sex, tumor size,vascular invasion, or metastasis. These results were similar to those published by Liu et al.27who concluded that neither serum nor tissue annexin A2 were useful biomarkers for the diagnosis of patients with HCC and cirrhosis, but may instead be a marker of liver cirrhosis. Moreover, Liu et al.27found no significant correlation between the levels of either serum or tissue annexin A2 and the tumor characteristics. In addition, El-abd et al.28and Ibrahim et al.29found no significant correlations between the levels of serum or tissue annexin A2 and the tumor clinicopathological characteristics.However, El-abd et al.28, Ibrahim et al.29, and Shaker et al.30found that serum levels of annexin A2 were significantly increased in patients with HCC compared with patients with chronic liver disease and the control group. No significant difference was detected between patient with chronic liver disease and the controls. This discrepancy from our results could be explained partly by the variability in the characteristics of included populations by Mohammad et al.31who found that levels of annexin A2 protein and mRNA were rarely found in both normal and chronic hepatitis liver tissues, but were overexpressed at both the transcriptional and translational levels in malignant and nonmalignant cirrhotic regions of HCC. Zhang et al.32who dynamically studied the proteins related to hepatic fibrogenesis in various stages (S0-1, S2, and S3-4) through plasma membrane proteomic technology, found that annexin A2 was highly elevated in S4 compared to that S0-1 and concluded that annexin A2 was a valid biomarker for noninvasive diagnosis of hepatic fibrosis. Elgezawy et al.33also found that the serum annexin A2 level was significantly elevated in liver fibrosis, liver cirrhosis, early and late HCC,but was not elevated in patients with chronic hepatitis compared with the control group (P < 0.01).
To the best of our knowledge, this study is the first to evaluate the α2δ1 subunit of VGCC as a potential biomarker for HCC diagnosis. The results revealed that the serum level of the α2δ1 subunit was highly promising as a single, noninvasive biomarker for the diagnosis and early detection of HCC, as it was significantly higher in patients with HCC compared to patients with cirrhosis and the normal control group (P < 0.001), with 100% sensitivity, 96% specificity,98% PPV, and 100% NPV, 98.7% accuracy when the ROC curve was used to select the optimal cut-off point (14.22 ng/dL), and an AUC of 0.977.
No statistically significant correlation was found between the serum level of the α2δ1 subunit and the clinicopathological features of patients.
VGCCs are functionally activated in non-excitable cells,furthermore, they contribute to Ca2+dependent signaling processes34. VGCCs are expressed in several cancers at both the gene and protein levels35, and are implicated in many of the hallmarks of cancer biology34, proposed by Hanahan and Weinberg36, which are: sustaining proliferative signaling pathways, escaping growth suppression, resistance to cell death, enabling replicative immortality, inducing angiogenesis, activating invasion and metastasis, and finally,enabling the hallmarks of reprogramming energy metabolism and evading destruction by immune response36. L-type channel voltage-gated calcium channels have been found in lymphocytes, although their functional role is not determined37. Buchanan & McCloskey34indicated the potential of repurposing calcium channel blockers for cancer therapy.
Zhao et al.22identified the α2δ1 subunit as a functional liver tumor - initiating cell marker. The α2δ1 subunit plays a crucial role in cell adhesion/extracellular signaling, which is not clearly related to traditional calcium channel functions23.The eradication of hepatic cancer stem cells is a target for the improvement of the outcome of patients with HCC38.Although pregabalin and gabapentin are known to bind to both the α2δ1 and α2δ2 subunits, which are present in the central nervous system and neuronal tissues39; the exact mechanism of action is not completely clear, but they undergo negligible metabolism (< 1% of the dose). Neither gabapentin nor pregabalin inhibits cytochrome P450 enzymes40, but no studies have previously reported their role as a blocker for α2δ1 in liver cells.
Conclusions
This study introduced the serum level of a hepatic cancer stem cell marker, the α2δ1 subunit of VGCC, as a potential novel biomarker for HCC, but larger, well planned phase III studies are crucial to validate the accuracy of the serum level of the α2δ1 subunit for the diagnosis of HCC.
Our results also revealed that the serum level of annexin A2 might not be an appropriate biomarker for the diagnosis of HCC in the presence of underlying cirrhosis, as no statistically significant difference was detected between patients with HCC and those with cirrhosis, but it might play a role in progression of chronic liver disease.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A.Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65: 87-108.
2.Ibrahim AS, Khaled HM, Mikhail NNH, Baraka H, Kamel H.Cancer incidence in Egypt: results of the national population-based cancer registry program. J Cancer Epidemiol. 2014; 2014: 437971
3.Hoshida Y, Fuchs BC, Bardeesy N, Baumert TF, Chung RT.Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J Hepatol. 2014; 61: S79-90.
4.Reichl P, Mikulits W. Accuracy of novel diagnostic biomarkers for hepatocellular carcinoma: An update for clinicians (Review). Oncol Rep. 2016; 36: 613-25.
5.Wang CY, Lin CF. Annexin A2: its molecular regulation and cellular expression in cancer development. Dis Markers. 2014;2014: 308976
6.Grieve AG, Moss SE, Hayes MJ. Annexin A2 at the interface of actin and membrane dynamics: a focus on its roles in endocytosis and cell polarization. Int J Cell Biol. 2012; 2012: 852430
7.Madureira PA, Hill R, Miller VA, Giacomantonio C, Lee PWK,Waisman DM. Annexin A2 is a novel cellular redox regulatory protein involved in tumorigenesis. Oncotarget. 2011; 2: 1075-93.
8.Cesarman GM, Guevara CA, Hajjar KA. An endothelial cell receptor for plasminogen/tissue plasminogen activator (t-PA). II.Annexin II-mediated enhancement of t-PA-dependent plasminogen activation. J Biol Chem. 1994; 269: 21198-203.
9.Zhang HJ, Yao DF, Yao M, Huang H, Wu W, Yan MJ, et al.Expression characteristics and diagnostic value of annexin A2 in hepatocellular carcinoma. World J Gastroenterol. 2012; 18:5897-904.
10.Yao HX, Zhang ZQ, Xiao ZQ, Chen YH, Li C, Zhang PF, et al.Identification of metastasis associated proteins in human lung squamous carcinoma using two-dimensional difference gel electrophoresis and laser capture microdissection. Lung Cancer.2009; 65: 41-8.
11.Shetty PK, Thamake SI, Biswas S, Johansson SL, Vishwanatha JK.Reciprocal regulation of annexin A2 and EGFR with Her-2 in Her-2 negative and herceptin-resistant breast cancer. PLoS One. 2012;7: e44299
12.Zhang Q, Ye ZY, Yang Q, He XJ, Wang HJ, Zhao ZS. Upregulated expression of annexin II is a prognostic marker for patients with gastric cancer. World J Surg Oncol. 2012; 10: 103
13.Yang T, Peng H, Wang J, Yang J, Nice EC, Xie K, et al. Prognostic and diagnostic significance of annexin A2 in colorectal cancer.Color Dis. 2013; 15: e373-81.
14.Spijkers-Hagelstein JAP, Pinhanços SM, Schneider P, Pieters R,Stam RW. Src kinase-induced phosphorylation of annexin A2 mediates glucocorticoid resistance in MLL-rearranged infant acute lymphoblastic leukemia. Leukemia. 2013; 27: 1063-71.
15.Menell JS, Cesarman GM, Jacovina AT, McLaughlin MA, Lev EA,Hajjar KA. Annexin II and bleeding in acute promyelocytic leukemia. N Engl J Med. 1999; 340: 994-1004.
16.Xu XH, Pan W, Kang LH, Feng H, Song YQ. Association of annexin A2 with cancer development (Review). Oncol Rep. 2015;33: 2121-8.
17.Waters KM, Stenoien DL, Sowa MB, Von Neubeck C, Chrisler WB,Tan R, et al. Annexin A2 modulates radiation-sensitive transcriptional programming and cell fate. Radiat Res. 2012;179: 53-61.
18.Swisher JFA, Khatri U, Feldman GM. Annexin A2 is a soluble mediator of macrophage activation. J Leukoc Biol. 2007; 82:1174-84.
19.Catterall WA, Leal K, Nanou E. Calcium channels and short-term synaptic plasticity. J Biol Chem. 2013; 288: 10742-9.
20.Davies A, Kadurin I, Alvarez-Laviada A, Douglas L, Nieto-Rostro M, Bauer CS, et al. The α2δ subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc Natl Acad Sci USA. 2010;107: 1654-9.
21.Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L,Dolphin AC. Functional biology of the α2δ subunits of voltagegated calcium channels. Trends Pharmacol Sci. 2007; 28: 220-8.
22.Zhao W, Wang LM, Han HB, Jin KM, Lin N, Guo T, et al. 1B50-1,a mAb raised against recurrent tumor cells, targets liver tumorinitiating cells by binding to the calcium channel α2δ1 subunit.Cancer Cell. 2013; 23: 541-56.
23.García K, Nabhani T, García J. The calcium channel α2δ1 subunit is involved in extracellular signalling. J Physiol. 2008; 586: 727-38.
24.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53: 1020-2.
25.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134: 1752-63.
26.Tangkijvanich P, Anukulkarnkusol N, Suwangool P, Lertmaharit S,Hanvivatvong O, Kullavanijaya P, et al. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. 2000; 31: 302-8.
27.Liu ZK, Ling Q, Wang JG, Xie HY, Xu X, Zheng SS. Annexin A2 is not a good biomarker for hepatocellular carcinoma in cirrhosis.Oncol Lett. 2013; 6: 125-9.
28.El-Abd N, Fawzy A, Elbaz T, Hamdy S. Evaluation of annexin A2 and as potential biomarkers for hepatocellular carcinoma. Tumor Biol. 2016; 37: 211-6.
29.Ibrahim AM, Hashem ME, Mostafa EF, Refaey MM, Hamed EF,Ibrahim I, et al. Annexin A2 versus Afp as an efficient diagnostic serum marker for hepatocellular carcinoma. J Gastroenterol Hepatol Res. 2013; 2: 780-5.
30.Shaker MK, Fattah HIA, Sabbour GS, Montasser IF, Abdelhakam SM, El Hadidy E, et al. Annexin A2 as a biomarker for hepatocellular carcinoma in Egyptian patients. World J Hepatol.2017; 9: 469-76.
31.Mohammad HS, Kurokohchi K, Yoneyama H, Tokuda M,Morishita A, Jian G, et al. Annexin A2 expression and phosphorylation are up-regulated in hepatocellular carcinoma. Int J Oncol. 2008; 33: 1157-63.
32.Zhang LJ, Peng X, Zhang ZQ, Feng YL, Jia XF, Shi YX, et al.Subcellular proteome analysis unraveled annexin A2 related to immune liver fibrosis. J Cell Biochem. 2010; 110: 219-28.
33.Elgezawy E, Khalaf M, Eldeen M, Abdelbaky L, Eldeek S. Expression of circulating annexin A2 in hepatic diseases and hepatocellular carcinoma. Ann Oncol. 2015; 26: iv6
34.Buchanan PJ, McCloskey KD. CaV channels and cancer: canonical functions indicate benefits of repurposed drugs as cancer therapeutics. Eur Biophys J. 2016; 45: 621-33.
35.Wang CY, Lai MD, Phan NN, Sun ZD, Lin YC. Meta-analysis of public microarray datasets reveals voltage-gated calcium gene signatures in clinical cancer patients. PLoS One. 2015; 10: e0125766
36.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100: 57-70.
37.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev.2015; 67: 821-70.
38.Nio K, Yamashita T, Kaneko S. The evolving concept of liver cancer stem cells. Mol Cancer. 2017; 16: 4
39.Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel α2-δ (alpha2-delta)subunit as a target for antiepileptic drug discovery. Epilepsy Res.2007; 73: 137-50.
40.Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010; 49: 661-9.
猜你喜欢
杂志排行
Cancer Biology & Medicine的其它文章
- The evolution of Epstein-Barr virus detection in nasopharyngeal carcinoma
- Dual-specificity phosphatase 6 (DUSP6): a review of its molecular characteristics and clinical relevance in cancer
- Silencing of syndecan-binding protein enhances the inhibitory effect of tamoxifen and increases cellular sensitivity to estrogen
- EGFR tyrosine kinase inhibitor HS-10182 increases radiation sensitivity in non-small cell lung cancers with EGFR T790M mutation
- Parkin protein expression and its impact on survival of patients with advanced colorectal cancer
- A new combined criterion to better predict malignant lesions in patients with pancreatic cystic neoplasms
