Preparation and Characterization of Nanostructured Lipid Carrier Loaded with UVA/UVB Filters
2018-02-20ZhangQianjieZhaoXiaoweiSongLiliOuWenhuaZhangWanPing
Zhang Qianjie, Zhao Xiaowei, Song Lili, Ou Wenhua, Zhang WanPing
School of Perfume and Aroma Technology, Shanghai Institute of Technology, China
Zhu Haiyang
Shanghai Ruxi Bio-Tech Co., Ltd., China
Abstract A new photoprotective system based on encapsulating UVA (butyl methoxydibenzoylmethane, BMBM)and UVB (octyl methoxycinnamate, OMC) filters into nanostructured lipid carriers (NLC) has been prepared to develop cosmetic formulations with effective UV protection. BMBM/OMC-loaded NLC was prepared by ultrasonication-homogenisation, and analysed by particle size, zeta potential (ZP), encapsulation efficiency (EE),scanning electron microscopy (SEM), fourier transform infrared spectroscopy (FTIR) and differential scanning calorimetry (DSC). Moreover, the UV protection property and photostability were investigated and compared with BMBM/OMC-conventional emulsions. The particle size and ZP of BMBM/OMC-loaded NLC were 310.24 nm and -33.6 mV, EE of BMBM and OMC were 85.46% and 99.32%. SEM, FTIR and DSC analysis confirmed BMBM and OMC entrapped in the lipid matrix core and the structure was stable during storage. Compared with conventional emulsion, BMBM/OMC-loaded NLC displayed perfect photo protection property in whole UV range. The photostability studies showed that the NLC can improve the photostability of sunscreens.
Key words nanostructured lipid carriers; butyl methoxydibenzoylmethane; octyl methoxycinnamate; photostability; synergistic effect

Introduction
Lipid nanoparticles have attracted increasing attention as an alternative colloidal system to micro-emulsions and polymeric nanoparticles.[1,2]Compared with other colloidal systems, such as polymeric nanoparticles and nanoemulsions, lipid nanoparticles possess many benefits such as low cytotoxicity, controlled drug delivery,great kinetic stability, excellent biocompatibility and biodegradability.[3-6]Solid lipid nanoparticles (SLN),the first generation of lipid nanoparticles, exhibit high crystallisation of solid lipids or solid lipid mixtures but also have some potential limitations, such as polymorphic transitions, limited drug payloads and drug expulsion during storage.[7,8]To overcome the limitations of SLN,a new generation of lipid nanoparticles, nanostructured lipid carriers (NLC), has been developed based on a mixture of solid and liquid lipids.[9-11]The choice of the liquid lipid plays a critical role because the active compounds must be solubilized and/or retained within the lipid matrix during storage.[12]The incorporation of liquid lipids in the structure inhibits crystallisation by mixing“spatially” different molecules, causing imperfections in the lattice that may accommodate the active ingredient.[13,14]In addition, NLC remain in solid state by controlling the liquid lipid content, therefore, controlled drug release properties for NLC can be achieved.
UV light can be divided into UVC (100~290 nm),UVB (290~320 nm), and UVA (320~400 nm). The skin exposure to UV radiation can result in a broad range of adverse effects, such as sunburn, photoaging,photoimmunosuppression and photocarcinogenesis.[15,16]In general, two basic types of sunscreen products were widely used in all types of topical products which are physical UV shielding agents and chemical UV absorber.Currently, chemical filters, depending on their chemical structures, are widely used as active ingredients in filters formulation. Butyl methoxydibenzoylmethane (BMBM)is the most efficient and widely used UVA filter.[17]However, BMBM undergoes marked decomposition and produces free radicals under sunlight exposure, leading to a decrease in its expected UV-protective power.[18]Octyl methoxycinnamate (OMC) is an oil-soluble chemical UV filters agent that absorbs UVB radiation. Some studies have reported that OMC is unstable and degrades when exposed to sunlight.[19,20]Therefore, new systems that improve the photo stability of organic UV filters are essential. The structure of BMBM and OMC was shown in Figure 1.

Figure 1. The structure of BMBM and OMC
The above mentioned NLC have been widely investigated as carrier systems to enhance chemical stability on isoliquiritigenin,[21]triamcinolone acetonide,[22]carotene,[23]docetaxel[24]and so on. The encapsulation of UV filters has also been reported. Montenegro et al.[25]prepared OMC and BMBM-loaded SLN via phase inversion temperature method. All prepared SLN showed a mean size ranging from 30 nm to 95 nm, but the loading capacity would be reduced when BMBM and OMC were loaded together. Puglia et al.[26]compared the influence of OMC loaded NLC onin vitropercutaneous absorption with nanoemulsion (NE) based formulations. Liu et al.[27]prepared solid lipid particles loaded with OMC and point out that SLN can be used as sunscreen carrier for improve the stability. Wang et al.[28]investigated the effect of solid lipid's structure on the physicochemical properties of NLC encapsulated with sun filter (BMBM) and the synergistic photoprotective effect of nanolipid carriers and UV filters were proved. However, the systematic studies on high-performance broad-spectrum sunscreen formulations which encapsulated UVA and UVB filters simultaneously were still lacked.
In this work, a new system based on encapsulating UVA (butyl methoxydibenzoylmethane) and UVB (octyl methoxycinnamate) filters into nanostructured lipid carriers (NLC) was prepared. The developed BMBM/OMC-loaded NLC characterized in terms of particle size, zeta potential, entrapment efficiency, SEM, FTIR,DSC and the preparation mechanism was discussed. In addition, the UV protection property and photostability were also investigated and compared with conventional emulsions.
Experimental
Materials
Hydrogenated castor oil (HR Cutina®HR, BASF)was used as solid lipid material of NLC. The lipophilic surfactant used for the formulation was cetyl stearic ether-20 (B2 Eumulgin®B2, BASF). The liquid lipid of caprylic/capric triglyceride (GTCC Myritol®312 BASF) was purchased from BASF. Organic UV filters,butyl methoxydibenzoylmethane (BMBM) and Uvinul MC80 (OMC, Octy lmethoxycinnamate) were provided by BASF and Alsland, respectively. Methanol, ethanol,tetrahydrofuran and perchloric acid were a kind gift of Tansoole. The water used throughout the experiments was deionized. All other reagents were of analytical grade and they were used as supplied.
Preparation of NLC
NLC was prepared using ultrasonic emulsification.Briefly, the oil phase was heated to 85 ℃, which consisted of 2.0% w/w solid lipid (HR), 6.5% w/w GTCC, 5.0% w/w B2 and organic UV filter (2% w/w BMBM and 5% w/w OMC). At the same time, the aqueous phase was prepared heated at the same temperature. The oil phase was then added to the aqueous phase with homogenising IKA®T-18 basic Ultra-Turrax®, Germany) at 14,000~15,000 rpm for 3 min. Finally, the emulsion was treated by a probetype sonicator (CP 750, Cole-Parmer, and Chicago, IL)at 400 W for 15 min. The prepared nanoemulsions were cooled down to 28℃ with continuously stirring (RW20,IKA, Staufen, Germany) at 900 rpm/min to obtain NLC.Conventional emulsion (CE) was composed of B2, GTCC and the equal amount sunscreen of BMBM and OMC as control.
Determination of particle size and zeta potential
Particle size (z-average diameter), polydispersity index (PI) and zeta potential (ZP) of all NLC were measured by Zetasizer Nano ZS (Malvern Instruments,Malvern, UK) at 25 ℃. The instrument performed particle sizing by means of a 4 MW laser diode operating at 670 nm. ZP was automatically calculated from the electrophoretic mobility based on Smoluchowski's equation:
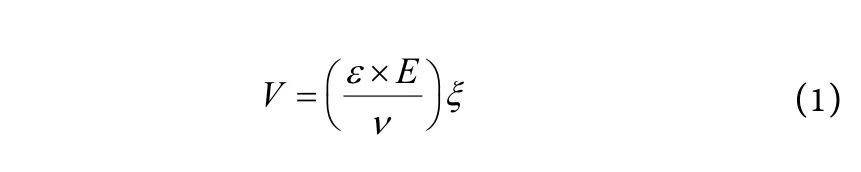
Wherevis the measured electrophoretic velocity,Eis the viscosity,ԑis the electrical permittivity of the®ξis the electric field.
The pH was in the range of 5.5~6.0 and the applied field strength was 20 V/cm. Before measurement, the samples were diluted 50 times with double distilled water.All measurements were performed in triplicate.
Encapsulation efficiency
A fixed quantity of NLC dispersion (2 mL) was placed in a centrifuge tube and centrifuged at 18,000 rpm for 30 min at room temperature (TGL-16aR). The lipid portion was isolated, and the absorbance of the filters in the supernatant was determined by reversed-phase highperformance liquid chromatography (HPLC). Samples were filtered and injected into the HPLC system. Agilent HPLC (Agilent 1260 series; USA) attached with a reversedphase C18 column (Fortis C18; 4.6 mm × 250 mm, 5 μm;Agilent, USA) was used for the assay. The mobile phase used consisted of methanol, tetrahydrofuran, deionised water and perchloric acid (25:45:30:5) at 1.0 μL·min-1isocratic flow. The temperature of the column and detection wavelength was set at 35 ℃ and 312 nm, respectively.
The amount of BMBM and OMC that can be incorporated into the particles were determined by calculating the encapsulation efficiency (EE) of BMBM and OMC according to the following equation:
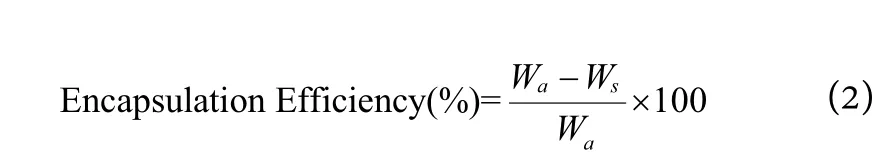
WhereWais the weight of filters added to the system,andWsis the mass of filters in the supernatant obtained after separation.
Scanning electron microscopy (SEM) analysis
Shape and surface morphology of the BMBM/OMC-loaded NLC were examined by scanning electron microscopy (SEM) (FEI Company, Quanta200 FEG,Holland). Prior to analysis, the sample was diluted with deionized water, placed on a double side carbon tape mounted onto an aluminum stud, and air-dried. Then,images were obtained at ×20,000 magnification at an excitation voltage of 5 kV.
Fourier Transform Infrared Spectroscopy(FTIR)
Fourier transform infrared spectroscopy of BMBM/OMC-loaded NLC, NLC, BMBM, OMC were measured with a VERTEX 70 FTIR spectrophotometer (Bruker,Germany) in the wavenumber from 4,000 to 600 cm-1under 25℃. The BMBM/OMC-loaded NLC and NLC were prepared in tablet using potassium bromide after freeze-drying.
Differential scanning calorimetry (DSC)investigations
The thermodynamic stability of NLC relies on their existing lipid modification. DSC functions on the principle that different lipid modifications possess different melting points and melting enthalpies. After heating, certain information about sample crystal lattice resolution can be shown in DSC curves, such as melting point, crystallisation order and co-crystal mixture.The crystalline index (CI [%]), which is defined as the percentage of the lipid matrix that has recrystallised during storage time, was calculated by applying the following equation:

WhereΔHis the melting enthalpy (J/g) that can be obtained from the area under the DSC curve of the melting endotherm.
Differential scanning calorimetry (DSC) analysis was performed using DSC Q2000 apparatus (TA Instruments,USA). The thermograms of Cutina®HR, B2, and lyophilized unloaded NLC, BMBM-loaded NLC, OMC-loaded NLC,BMBM/OMC-loaded NLC were recorded. Moreover, the samples of BMBM/OMC-loaded NLC were respectively carried after storage for three months and six months.Samples (2~5 mg) sealed in standard aluminum pans were kept under isothermal condition at 2 ℃ for 10 min.An empty pan was used as a reference. DSC scans were recorded at heating rate of 10℃·min-1. All samples were heated from 0℃ up to 200℃.
In Vitro Sun Protection Factor Measurement
Anin vitrostudy was performed to evaluate the Sun Protection Factor (SPF) of the nanoparticle lipid nanoparticles and conventional emulsion. SPF was assessed by the Optometrics SPF-290S Analyzer. The samples were prepared by spreading 100 mg of each formulation over a Transpore®tape (70.7 × 70.7 mm)having an area of 50 cm2to obtain a film of 2 mg/cm2, as specified by the U.S. Food and Drug Administration.[29]Each sample was exposed to axenon arc solar simulator, and the Analyzer performed scans in 12 different spots on the Transpore®tape substrate. Each scan takes a transmittance(T) measurement every 2 nm from a wavelength ranging from 290 to 400 nm. The method forin vitrodetermination of SPF of sunscreens is based on Diffey and Robson theory:The SPF value is calculated by Eq. (4).[30]


WhereEλis the spectral irradiance of sunlight at wavelength λ;Bλis the erythemal effectiveness at wavelength λ;MPFλthe monochromatic protection factor for selected wavelength (the difference between the spectrum of measured sample applied on support and support spectrum).
Ultraviolet-visible absorption spectroscopy
The ultraviolet spectrograph of samples about BMBM/OMC-loaded NLC and BMBM/OMC-conventional emulsion were tested by solution method. The absorption spectra of samples were investigated using UV-spectrophotometry (Shimadzu UV Spectrophotometer)in the ultraviolet range of 190~400 nm. 1 mL of BMBM/OMC-loaded NLC and BMBM/OMC-conventional emulsion was diluted 100 times with deionized water before irradiation and the absorbance were recorded. All measurements were performed in triplicate.
In vitro photostability studies
Anin vitrostudy was performed to evaluate the photoprotective effect of the lipid nanoparticle and conventional emulsion against UV radiation. The photodegradation kinetics can be expressed by Eq. (5).[31]

WhereCtis the concentration of time t,C0is the initial concentration of BMBM/OMC,Kis the photodegradations rate constant,t isthe time in hours.
Each formulation was diluted with deionised water 100 times and spread in a glass surface vessel sealed with transparent film. The samples were then placed in a constant temperature and constant humidity box(Climacell 222, MMM Group, Germany) equipped with UV lamps. The applied UV energy was equivalent to 20 minimal erythema doses, which is considered representative of daily solar emission. The samples were obtained after irradiation at pre-determined time intervals (2, 4, 6, 8, 10 and 12 h). After diluting with ethanol, the UV filters were quantified by UV spectroscopy.
Results and discussion
Preparation of BMBM/OMC-loaded NLC
In this study, Cutina®HR (Hydrogenated castor oil)was chosen for the solid lipid matrix, GTCC (caprylic/capric triglyceride) was used as the liquid lipid of the matrix which made the NLC different from the formulation of solid lipid nanoparticles (SLN) and the non-toxic, non-ionic surfactant, cetyl stearic ether-20 (B2)was used as surfactant.
The BMBM/OMC-loaded NLC was successfully prepared by ultrasonic emulsification method. The average particle size and zeta potential of obtained BMBM/OMC-loaded NLC were shown in Figure 2.Particle size of the colloidal dispersion was 310.24 nm and the polydispersity index (PI) was 0.237±0.006. The analysis of the ZP, which is the electric potential at the plane of shear, is a useful tool to predict the physical storage stability of colloidal systems. The nanoparticles had the higher ZP value, indicating the better stability of this colloidal system.[32]An absolute value above 30 mV usually indicates good stability of the colloid dispersion.ZP values higher than -30 mV showed good physical stability, being optimized when they reach approximately-60 mV, exhibiting a very good physical stability during the shelf-life. In the present study, the ZP value of BMBM/OMC-loaded NLC was -33.6 mV, indicating the NLC should possess a good physical stability. It could attribute that the particle aggregation was partly avoided by electrostatic repulsion. In addition, the encapsulation efficiency of BMBM and OMC were 85.46% and 99.32%respectively.

Figure 2. The particle size (left) and zeta potential (right) distribution of the BMBM/OMC-loaded NLC
Morphology study of BMBM/OMC-loaded NLC
The morphology of BMBM/OMC-loaded NLC was determined by SEM (as shown in Figure 3). As indicated in Figure 3, particle sizes of BMBM/OMC-loaded NLC were consistent with the results obtained by Dynamic Light Scattering (DLS) characterization and depicted a mono-dispersed spheroid-like appearance with a distinct boundary between each particle. As indicated in SEM image depicted BMBM/OMC-loaded revealed round and homogeneous shading with a distinct boundary between each particle. There was no drug crystal or aggregation of particles visible in the graph.

Figure 3. SEM photographs of the BMBM/OMC-loaded NLC
FTIR investigation
FTIR was used to investigate the intermolecular interaction between BMBM, OMC and NLC. The infrared spectra of BMBM, OMC, unloaded NLC and BMBM/OMC-loaded NLC were obtained and presented in Figure 4. Pure BMBM exhibited obvious absorption peaks at 1,593~1,459 cm-1and 1,258 cm-1were assigned to benzene and C-O-C, respectively.[28]FTIR spectrum of pure OMC displayed a stretching vibration band of C=O at 1,709 cm-1;benzene skeleton vibration bands at 1, 604, 1,513 and 1, 462 cm-1; and a para-orientation peak of CH2 at 829 cm-1.[33]By contrast, the corresponding characteristic peaks for BMBM/OMC-loaded NLC were observed at 1,710, 1,603, 1,512, 1,463 and 835 cm-1. Except for the C=O stretching vibration of BMBM/OMC-loaded NLC, in which the spectrum diminished to a small extent possibly because of the reduction in sharpness crystallinity of the drug, other spectra were simply regarded as the superposition of BMBM and OMC. The FTIR spectrum of BMBM/OMC-loaded NLC showed no significant shift in all the characteristic bands described above. This result suggested the absence of an interaction between components in the formulation, thereby confirming BMBM and OMC entrapped in the lipid matrix core.Compared with BMBM/OMC-loaded NLC, the unloaded NLC did not exhibit the same characteristic peaks.

Figure 4. FTIR spectrum of the BMBM, OMC, NLC and BMBM/OMC-loaded NLC
DSC analysis
DSC is frequently used to measure heat loss or gain resulting from physical or chemical changes within a sample as a function of temperature. It has been used to characterise the state and crystallinity of drug in the compounds and NLC.[34,35]
The DSC thermograms of solid components which are HR, B2 and BMBM are shown in Figure 5A. The distribution of the active compounds in the lipid nanoparticles is related to the melting of the solid lipid and active compound. The calorimetric curve of HR bulk was characterised by a broad endothermic peak at 40.68℃ followed by a main peak centred at about 73.84℃(ΔHHR=132.65 J/g) because of melting. The DSC thermogram of BMBM showed a sharp endothermic peak at 72.14℃. The melting point of BMBM was close to the solid lipid of HR. Therefore, BMBM filter is mainly distributed in two forms: homogenously distributed in matrix and enriched on the shell. In the first form, the active agent is molecularly dispersed evenly in the particle matrix.For the second form, the lipid and active agent precipitate simultaneously in the outer shell of the particles. A similar thermal profile was recorded for the emulsifier B2. The DSC profile showed an endothermic peak at 36.62℃.
The thermal behaviours of unloaded NLC, BMBM-loaded NLC, OMC-loaded NLC and BMBM/OMC-loaded NLC are shown in Figure 5B. All DSC thermogram presented a sharp endothermic peak with a broad shoulder peak on the right due to the melting of the solid components. Compared with unloaded NLC, the endothermic peak of OMC-loaded NLC showed less intense and shifted towards lower temperature because of the distribution of OMC in the nanoparticles. The addition of OMC also resulted in a decrease in CI from 40.18% to 32.56%. In contrast, encapsulated BMBM into NLC resulted in an increase in CI because of the crystalline nature of BMBM at room temperature. The BMBM/OMC-loaded NLC showed similar thermal behaviour with the unloaded NLC. Only the presence of BMBM and OMC induced broadening of peaks(Tonset,loadedNLC=9.51℃, Tonset,unloadedNLC=20.49℃), which could be attributed to the imperfections in the lipid crystal lattice. These imperfections decreased the binding forces between the molecules of the lipid matrix.
To evaluate the stability of this system, the BMBM/OMC-loaded NLC was stored at 25℃ for three and six months. As shown in Figure 5C, the thermal behaviours did not change significantly after three and six months.Thus, the structure of BMBM/OMC-loaded NLC was stable during storage, and polymorphic transition did not occur. The CI was 44.81% after six months, which indicates that the particles remained in the solid state during this period.
In vitro UV absorptive properties/UV protection properties

Figure 5. DSC thermograms of (A) solid component of HR,BMBM, B2; (B) Blank NLC, BMBM-loaded NLC, OMC-loaded NLC, BMBM/OMC-loaded NLC; (C) BMBM/OMC-loaded NLC: storaging for 1 day, 3 months and 6 months
Müller et al.[35]found that SLN and NLC can act as physical UV blockers themselves and improve UV protection. In the present study, the UV absorption of BMBM/OMC-loaded NLC was compared with a conventional emulsion containing 2% BMBM and 5%OMC and the UV spectrograph was shown in Figure 6.The characteristic absorption peaks of BMBM and OMC were at 362 and 312 nm, respectively. As shown in Figure 6, the photo protection effect of BMBM was enhanced by about 20% after encapsulation in the lipid nanoparticles and the increase in the photoprotection effect of OMC was about 50%. This behaviour was attributed to the synergistic effect caused, firstly, by the incorporation of BMBM and OMC into lipid nanoparticles and, secondly,by the NLC particles which could shield UV radiation effectively. Further, the solid lipid protected BMBM and OMC from degradation and enhances the efficiency of UV filters, simultaneously, the solid lipid particles can enhance the sunscreen value by acting as physical UV blockers reflecting or refraction of the UV light.

Figure 6. UV absorption of BMBM/OMC-loaded NLC and BMBM/OMC-conventional emulsion (CE), NLC and CE
Since one of the most important criteria for the assessment of a sunscreen product performance is the sun protection factor (SPF), and the SPF values cannot be predicted from simple UV spectroscopic analysis.Therefore, this parameter was determined invitro in the examined formulations. BMBM/OMC-loaded NLC showed significantly higher invitro SPF (SPF=41.53±2.72)when compared with BMBM/OMC-conventional emulsion (SPF=33.43±1.64), confirming the results from UV absorption spectrum. This fact indicated better UV blocking property could obtain by introducing lipid nanoparticles which closely arranged on the skin surface and effectively blocked the UV transmission.
In vitro photostability of UV filters
The photostability evaluation of the UV filters loaded into lipid nanoparticles is a prerequisite to develop efficient and safe sunscreen formulations. The cream formulations based on BMBM/OMC-loaded NLC and conventional emulsion were subjected to a photochemical UV irradiation at a low energy that simulates the solar energy during the middle of the day. The spectral behaviours at 312 and 362 nm were shown in Figure 7, respectively.
As shown in Figure 7, the photodegradation of BMBM/OMC encapsulated in conventional emulsion and NLC under UV irradiation both followed the first order kinetic and the obtained lines followed the Eq.(5) reported previously. Compared with conventional emulsion, the degradation of BMBM/OMC encapsulated in NLC was slower which indicated the UV filters loaded in NLC were more photostable. This could be explained by BMBM and OMC underwent cis-trans isomerisation,which can be considered a highly efficient way of dispersing absorbed energy during UV irradiation and the solid lipid matrix may inhibit this reaction.

Figure 7. Photodegradation of BMBM/OMC-loaded NLC and BMBM/OMC-conventional emulsion (CE) upon irradiation: 362nm (left); 312nm (right)
Conclusion
In this study, a new system based on nanostructured lipid carriers encapsulated with UVA filter (butyl methoxydibenzoylmethane) and UVB filter (octyl methoxycinnamate) have been developed. The particle size of BMBM/OMC-loaded NLC was 310.24 nm and the polydispersity index (PI) was 0.237±0.006. The ZP value of obtained NLC was -33.6 mV which indicated the NLC should possess a good physical stability. SEM analysis showed the uniformly spherical shape and nanoscale dimensions of the BMBM/OMC-loaded NLC. FTIR analysis revealed the absence of an interaction between components in the formulation, thereby confirming BMBM and OMC entrapped in the lipid matrix core,confirmed by DSC study. By encapsulating BMBM and OMC into NLC, the developed NLC system provided perfect photo protection property and increased the SPF from 33.43±1.64 to 41.53±2.72. This improvement could be attributed to the synergistic effect caused by the incorporation of UV filters into lipid nanoparticles and then by the UV protection effect of the lipid matrix.Meanwhile, the developed NLC also provided a stabilizing effect of BMBM and OMC under exposure to artificial UV radiation.
Acknowledgement
Funding: This research was supported by Base Construction Program of Shanghai Institute of Technology: Breeding of new variety and Highyield Cultivation Technique of Fragrant Plants(3921NH166035)

杂志排行
China Detergent & Cosmetics的其它文章
- Alibaba's Tmall and L'Oréal China Partner Up to Launch New Campaign for Men's Grooming Products
- Pechoin Wins the Innovation Golden Award at the 30th IFSCC Congress in Munich
- Coming Soon on Tmall: Boots Makes First Move into China
- IFF's New Naturals Lab in China Is the First Outside of the US
- 2018 CCIA Annual Meeting &Industry Convention Successfully Held in Zhuhai
- Major Industry Events
