改良法建立内膜损伤致大鼠颈动脉狭窄模型
2016-12-29张耀雷呼永和
张耀雷,李 昆,张 彦,杨 炯,呼永和
改良法建立内膜损伤致大鼠颈动脉狭窄模型
张耀雷,李 昆,张 彦,杨 炯,呼永和
目的改良球囊致内膜损伤建立大鼠颈动脉狭窄模型,并对其发生机制作初步探究。方法42只SD大鼠随机分为模型组(n=21)和假手术组(n=21),模型组利用10 m l注射器针头模拟球囊抽拉致内膜损伤建立颈动脉狭窄模型;假手术组除针头抽拉损伤外,其余处理与模型组一致。伊文思蓝染色观察两组(每组3只)术侧颈总动脉内膜损伤差异;分别于术后1、2及3 w取两组颈总动脉(每组每次6只)行HE染色,观察血管狭窄变化;将2 w颈总动脉行α-smooth muscle actin免疫组化染色及二氧乙啶(DHE)测量活性氧(ROS)含量。结果伊文思蓝染色指示,针头抽拉区域内膜损伤。HE染色显示,模型组术后1 w时内膜开始增殖,2 w时内膜快速增殖达到1 w时的6.1倍,血管狭窄形成;3 w时内膜缓慢增值达到1 w时的6.8倍。α-smooth muscle actin染色指示新生内膜大部分为平滑肌细胞(VSMCs)。与假手术组相比,模型组ROS表达量急剧升高(P<0.01)。结论利用注射器针头抽拉成功建立大鼠颈动脉狭窄模型,其机制可能是内膜损伤使ROS含量升高,诱导VSMCs由中膜迁移至内膜并恶性增殖,从而导致颈动脉狭窄发生。
注射器针头;颈动脉狭窄;大鼠模型;ROS
经皮穿刺腔内冠状动脉成形术(percutaneous transluminal coronary angioplasty,PTCA)术后血管再狭窄(restenosis,RS)是阻碍冠心病介入治疗的重大难题,建立一种操作时间短、手术材料简单、稳定性高、重复性好且可控制狭窄程度的血管损伤狭窄模型,对于研究RS有重要意义[1]。以往建立的狭窄模型特别是大鼠模型,很少能同时具有上述所有的优点[2-3]。本研究采用改良法成功建立了一种操作时间短、手术材料简单及重复性好的内膜损伤致颈动脉狭窄模型,并对其狭窄发生机制作了初步探究。
1 材料与方法
1.1 实验材料
1.1.1 实验动物 SPF级SD大鼠42只,雄鼠,体重200 g,购自成都达硕生物科技有限公司,实验动物许可证号:SCXK(川)2008-24。应用随机数字表法随机分为:模型组(n=21)和假手术组(n=21)。按实验动物使用的3R原则给予人道的关怀。
1.1.2 主要试剂及器材 HE染色试剂盒(Beyotime),免疫组化试剂盒(Beyotime),ROS荧光探针(Invitrogen),Rabbit anti-α-smooth muscle actin(Boster);10 ml一次性使用无菌注射器(36 mm×0.8 mm,山东威高),显微手术器械(弯镊、直镊、剪刀)。
1.2 实验方法
1.2.1 颈动脉狭窄模型建立 模拟Langberg等[4]的方法,4%水合氯醛1 ml/100 g腹腔注射麻醉大鼠,沿颈部正中线剪开皮肤(3~4 cm)。如图1、2示,逐层钝性分离肌肉,暴露左侧颈总动脉,向远心端寻找颈内、外动脉分叉。结扎颈外动脉远心端,颈内动脉远心端打活结,夹闭颈总动脉近心端,颈外动脉结扎处与颈内、外动脉分叉处中间垂直切口,大小为血管横截面1/3到1/2。模型组选取10 ml注射器针头,从该切口插入,与血管内膜紧密贴合,保持与血管平行,缓慢进入颈总动脉至A1处;然后将针头缓慢退回切口,如此来回抽拉3次,每次抽拉时针尖顺时针旋转120°。假手术组除不插刀抽拉针头外,其余处理和模型组一致。结扎切口,打开活结及动脉夹恢复供血。
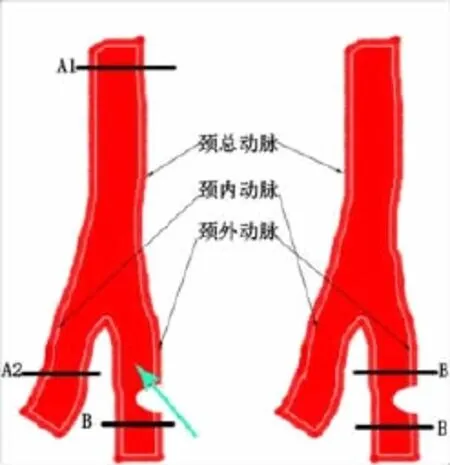
图1 模型建立示意图
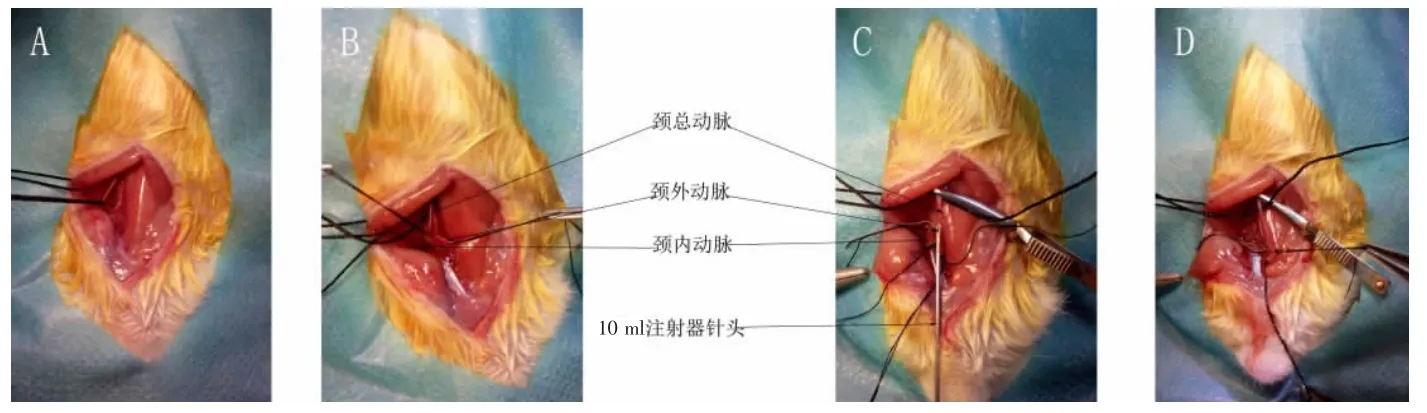
图2 模型建立过程略图
1.2.2 损伤程度及部位观察 两组各取3只大鼠,术后10 min,打开缝线向颈总静脉注射1 ml伊文思蓝,1 min后取下颈总动脉,用生理盐水冲洗3次,纵向切开血管,比较两组内皮损伤程度及部位差异。
1.2.3 血管组织形态学观察 分别在术后1、2、3 w,两组各6只大鼠,空气栓塞法处死大鼠,取下术侧颈总动脉,生理盐水冲洗血管,每个时间点留存3只大鼠颈总动脉标本做冰冻切片,其余放入4%甲醛固定过夜。固定的颈动脉标本用石蜡包埋并切片,每只随机取6张行HE染色,光学显微镜(200×)下观察血管新生内膜增殖变化,Image-Pro Plus(Media Cybernetics)测量新生内膜、中膜面积比。
1.2.4 免疫组化检测α-actin水平 两组各3只大鼠颈动脉标本石蜡切片,每只随机取6张常规脱蜡复水,新鲜3%H2O2孵育30 min。采用碱修复,电磁炉煮沸5 min,常温冷却5 min再煮沸5 min,冷却至常温。5%BSA,37℃孵育1 h,Rabbit anti-α-smooth muscle actin 1:800,4℃孵育过夜,羊抗兔二抗1∶1000,37℃孵育30 min,DAB显色统一10 s,常规苏木素复染核及封片。光学显微镜(200×)下观察血管α-actin分布,Image-Pro Plus(Media Cybernetics)检测α-actin水平。
1.2.5 免疫荧光检测活性氧(ROS)水平 留存的两组各3只大鼠颈总动脉冰冻切片,采用5%BSA、37℃封闭30 min,将DHE终浓度调整为10 μM,37℃孵育60 min。PBS洗3次,每次5 min。荧光显微镜(200×)下488 nm波长激发,Image-Pro Plus(Media Cybernetics,Silver Spring,MD)测量阳性区域表达比例。
1.3 统计学方法 应用SPSS19.0软件进行统计分析,计量资料以均数±标准差表示,组间比较采用独立样本t检验,P<0.05为差异有统计学意义。
2 结果
2.1 伊文思蓝观察内皮损伤结果 如图3所示,蓝色深浅代表损伤程度差异。模型组蓝色范围大且着色很深,自颈内、颈外动脉分叉处(图中红色箭头指示)向颈总动脉近心端延伸,损伤部位与针头拖动位置一致;假手术组颈总动脉两端离断处少量着色,其余部位着色较浅。

图3 针头抽拉后内膜损伤差异(伊文思蓝染色)
2.2 HE染色结果 从图4可见,针头损伤后1 w,内膜开始增厚 (内膜/中膜为0.7±0.02);2 w时内膜显著增加(内膜/中膜为4.3±0.12),显著厚于1 w时(P<0.01);3 w时内膜继续缓慢增厚 (内膜/中膜为4.8±0.16),显著厚于2 w时(P<0.05)。3个时间点颈总动脉中膜弹性纤维(中膜内深红色条纹)完整。

图4 颈动脉损伤后新生内膜变化(HE染色,×200)
2.3 免疫组化α-actin染色结果 从图5可见,假手术组中膜细胞完全着色;模型组中膜着色且新生内膜细胞大部分着色,分布均匀。

图5 术后2 w鉴定新生内膜中VSMCs成分(免疫组化染色,×200)
2.4 免疫荧光检测ROS含量结果 红色为ROS表达区域,实验结果显示,模型组ROS比例为4.2±0.04显著高于假手术组的1.0±0.05(P<0.01,图6)。

图6 术后2 w模型组与假手术组ROS表达水平 (DHE染色,×200)
3 讨论
PTCA术后易导致血管再狭窄,严重的阻碍介入手术的发展[5]。因此,建立能够很好反映血管损伤至狭窄的动物模型显得十分必要[6]。Van Osselaer等[7]用硅橡胶圈放置于颈动脉旁诱导内膜增厚,虽然成功建立家兔颈动脉狭窄模型,但没有硅橡胶圈标准的规格(大小、长短、形状)可参考,重复性不好。沈长银和Limin[8-9]等用氮气栓塞颈总动脉联合高脂饲料(1.5%胆固醇)喂养成功建立家兔颈动脉狭窄模型,但手术复杂,且狭窄区域不好控制差异很大。有研究利用电刺激颈动脉外膜,间断恒定脉冲联合高胆固醇喂养28 d成功建立家兔颈动脉狭窄模型,但操作复杂且成模率低[10]。目前运用最多的是球囊损伤法[11],利用导丝将球囊送入颈总动脉,球囊充满2~4个大气压反复拖动3次成功建立大鼠颈动脉狭窄模型。但此法球囊消耗很快,价格昂贵,且易造成血管中膜弹性纤维断裂。
本研究采用改良Tsuruta等[12]的球囊损伤法,将球囊换成大小适中的注射器针头,结果发现10 ml注射器针头正好与成年SD大鼠(200 g)颈动脉内膜贴合,且不损伤血管中膜弹性纤维。术后伊文思蓝染色,伊文思蓝与损伤内皮细胞循环蛋白结合而着色,附着于血管内壁,但完整的内皮可阻止这两者的结合,结果显示针头拖动部位血管内膜损伤。HE染色显示,1 w时内膜开始增殖,2 w增殖速度最快,3 w增殖仍缓慢增加,3个时间点血管中膜弹性纤维完整,提示针头大小适中,损伤后新生内膜增殖速度是先慢后快再变慢的趋势。Anti-α-smooth muscle actin特异的结合血管内平滑肌细胞肌动蛋白,用于指示和鉴定VSMCs分布及含量。利用α-actin对损伤部位颈动脉染色,发现新生内膜增殖主要成分为VSMCs。ROS介导多种疾病(恶性增殖、迁移、炎症等)发生,尤其是在RS过程中具有关键作用[13-14]。已有研究表明,ROS可以活化细胞外信号转导激酶、C-Jun N-末端激酶、p38丝裂原活化的蛋白酶通路,进而激活凋亡蛋白酶激活因子。同时还可以通过调节VSMCs胞外基质降解,导致VSMCs的恶性增殖和迁移[15-16]。术后2 w模型组 ROS含量显著高于假手术组(P<0.01),提示针头损伤导致ROS升高,可能使VSMCs由中膜迁移至内膜,并恶性增殖导致血管狭窄发生。
由于针尖是斜面,为避免损伤后血管新生内膜的偏心性增殖出现,每抽拉1次就换个方向继续抽拉;针尖锋利,抽拉时一定缓慢进行,且一直保持颈总动脉处于拉直状态,防止直接穿破血管;术后无需高脂或高胆固醇喂养;整个过程时间短,操作简单,材料造价便宜。
综上所述,采用改良法成功建立大鼠颈动脉狭窄模型,模型操作时间短、手术材料简单且重复性好。形成颈动脉狭窄的机制可能是内膜损伤致ROS含量上升,诱导胞内信号通路,使VSMCs由中膜向内膜迁移并恶性增殖。
[1] Lee Jong Y ung,Lee Cheol Whan,Kim WonJang,et al. Antiatherosclerotic effects of the novel angiotensin receptor antagonist Fimasartan on plaque progression and stability in a rabbit model:a double-blind placebo-controlled trial[J].Journal of Cardiovascular Pharmacology,2013,62(2):229-236.
[2] Zhang G,Li M,Li L,et al.The immunologic injury composite with balloon injury leads to dyslipidemia:a robust rabbit model of human atherosclerosis and vulnerable plaque [J].Biomed Research International,2011,2012(4):249129.
[3] Hilda Merino,Sampath Parthasarathy,Dinender K Singla.Partial ligation-induced carotid artery occlusion induces leukocyte recruitment and lipid accumulation-a shear stress model of atherosclerosis[J].Molecular&Cellular Biochemistry,2012,372 (1-2):267-273.
[4] Langberg CW,Solheim S,Hagen S.Can radiotherapy reduce the frequency of restenosis after coronary angioplasty[J]?Tidsskrift for Den Norske Legeforening Tidsskrift for Praktisk Medicin Ny Rekke,2000,120(6):707-710.
[5] Bauters C,Meurice T,Hamon M,et al.Mechanisms and prevention of restenosis:from experimental models to clinical practice[J]. Cardiovascular Research,1996,31(6):835-846.
[6] Lee JY,Lee CW,Kim WJ,et al.Antiatherosclerotic effects of the novel angiotensin receptor antagonist Fimasartan on plaque progression and stability in a rabbit model:a double-blind placebo-controlled trial[J].Journal of Cardiovascular Pharmacology, 2013,62(2):229-236.
[7] I Van Osselaer N,Van Put D,De Meyer GR.Role of polymophonuclear leukocytes in collar-induced intimal thickening in the rabbit carotid artery[J].Arterioscler Thromb Vasc Boil, 1998,18(6):915-921.
[8] 沈长银,石蓓,赵然尊,等.兔颈动脉粥样硬化狭窄动物模型的制备[J].四川大学学报:医学版,2009,40(5):923-926.
[9] Limin Ren,Caijin Li,Fengtao Fan,et al.Developing a rabbit model of neointimal stenosis and atherosclerotic fibrous plaque rupture [J].Journal of Tehran Heart Center,2011,6(3):117-125.
[10] Zhuang Z,Khurana R,Bhardwaj S.Angiogenesis-dependent and independent phases of intimal hyperplasia[J].Circulation,2004, 110(16):2436-2443.
[11] 崔丽,张恩园,李广平,等.兔颈动脉球囊损伤后动脉内膜、中膜厚度及面积的时相性变化[J].山东医药,2015,55(17):26-28.
[12] Tsuruta W,Yamamoto T,Suzuki K,et al.Simple new method for making a rat carotid artery post-angioplasty stenosis model[J]. Neurol Med Chir(Tokyo),2007,47(11):525-529.
[13] San Martín Alejandra,Kathy K Griendling.Redox control of vascular smooth muscle migration [J].Antioxidants&Redox Signaling,2010,12(5):625-640.
[14] Satoh K,Nigro P,Berk BC.Oxidative stress and vascular smooth muscle cell growth:a mechanistic linkage by cyclophilin A[J]. Antioxidants&Redox Signaling,2009,12(5):675-682.
[15] Svineng G,Ravuri C,Rikardsen O,et al.The role of reactive oxygen species in integrin and matrix metalloproteinase expression and function[J].Connective Tissue Research,2008,49(49):197-202.
[16] Weiwei Yin,Eberhard O Voit.Function and design of the Nox1 system in vascular smooth muscle cells[J].Bmc Systems Biology, 2013,7(1):1-20.
Establishment of model for carotid artery stenosis caused by intimal injury in rats by modified method
Zhang Yaolei1,Li Kun1,Zhang Yan2,Yang Jiong2,Hu Yonghe31.Central Laboratory,General Hospital of Chengdu Military Command, Chengdu,Sichuan,610083,China;2.Department of Cardiology,General Hospital of Chengdu Military Command,Chengdu,Sichuan, 610083,China;3.Department of TCM,General Hospital of Chengdu Military Command,Chengdu,Sichuan,610083,China
Objective To establish a model for carotid artery stenosis caused by intimal injury in rats by modified method and to preliminarily explore the occurrence mechanism.MethodsA total of 42 SD rats were randomly divided into two groups:the model group and the sham-operation group(n=21,respectively).A model for carotid artery stenosis was established in the model group by use of a 10 m l syringe needle to simulate the intimal injury caused by balloon drawing;except for needle drawing injury,other treatments in the sham-operation group were the same as that in the model group.Evans blue stain was used to observe the difference in the common carotid artery injury on the operation side in the two groups(three rats in each group);the cephalic artery in the two groups (six rats in each group)was sampled one,two and three weeks after the operation for HE stain to observe the change of angiostenosis; the two-week cephalic artery was received α-smooth muscle actin immunohistochemical staining and DHE to measure the content of ROS.ResultsEvans blue stain showed that intimal injury was caused in the needle drawing area.HE stain indicated that the intima began to increase within one week after the operation in the model group and increased to 6.1 times of that in one week upon two weeks,and angiostenosis is formed;the intima slowly increased to 6.8 times of that in one week upon three weeks.α-smooth muscle actin stain indicated that most of the new intima was VSMCs.Compared with the sham-operation group,the ROS in the model group increased greatly(P<0.01).ConclusionCarotid artery stenosis model in rats may be easily established by drawing of syringe needle. The mechanism may be that the intimal injury causes the increase of ROS,induces VSMCs to migrate from the media to the intima and proliferate malignantly,causing carotid artery stenosis.
syringe needle;carotid artery stenosis;rat model;ROS
R 543.4
A
1004-0188(2016)12-1409-04
10.3969/j.issn.1004-0188.2016.12.016
2016-05-15)
全军医学科技“十二五”重点项目(BWS11J067)
610083成都,成都军区总医院中心实验室(张耀雷,李 昆),心内科(张 彦,杨 炯),中医科(呼永和)
呼永河,E-mail:huyonghe@vip.126.com
