Synthesis and Thermal Properties of 1,1′-Dioxide-5,5′-azotetrazole Dipotassium Salt
2016-05-08XIAOXiaoYAOErgangLIUQingSUHaipengDINGKeweiGEZhongxue
XIAO Xiao, YAO Er-gang,2, LIU Qing, SU Hai-peng, DING Ke-wei, GE Zhong-xue
(Xi′an Modern Chemistry Research Institute, Xi′an 710065, China; 2. Science and Technology on Combustion and Explosion Laboratory, Xi′an 710065, China)
1 Introduction
Recently, attractive strategies in the design of new high-performaning energetic materials have fallen into a major catagory, ring- or cage-shaped compound with high heats of formation[1-3]. For energetic materials based on this strategy[4-10], the tetrazole-N-oxides has been found to occupy the ideal middle properties including high explosive performane and superior stablities at the same time. Therefore, the tetrazole-N-oxide unit can be used as precursor for the preparation of novel energetic materials which possess high density releasing plenty of energy and gasess upon decomposition or explosion[11-16. For example, the recent work from Niko Fischer[16], tetrazole-N-oxides have been used to prepare dihyeroxylammonium bistetrazolate-1,1′-dioxide(TKX-50), a useful explosive compound with performane exceeding that of HMX. Furthermore, Dennis Fischer et al[17]reported the synthesis and properties of 1,1′-dioxide-5,5′-azotetrazole dipotassium salt, which possessed superior calculated detonation properties and presents superior stability towards thermal and machanical stimulation. In this work, we reported the synthesis and characterization of 1,1′-dioxide-5,5′-azotetrazole dipotassium salt and the reaction mechanism was hypothesized for the first time in nation,as well as the thermal performance was studied by DSC and TG-DTG in this article.
2 Synthesis and Characterization
2.1 Materials and Instruments
All chemicals were of reagent-grade quality obtained from commercial sources and used without further purification.
1H NMR and13C NMR spectra were recorded on a Bruker AV-800 spectrometer. FT-IR(KBr) spectra were recorded on a PerkinElmer FT-IR spectrometer, and mass spectra were collected on a HP5989B mas spectrometer. Decomposition points were determined by differential scanning calorimetry(DSC) on a Linseis DSC-PT10 at a heating rate of 5 ℃·min-1. Thermogravimetry-derivative thermogravimetry(TG-DTG) were carried out on PerkinElmer Pyris-Ⅰ thermogravimetric analyzer with a heating rate of 5 ℃·min-1from 50 ℃ to 500 ℃ under N2atmosphere.
2.2 Synthesis and Characterization
1,1′-Dioxide-5,5′-azotetrazole dipotassium salt was synthesized via two-step reactions of azido-oximes-cyclization and oxidation-coupling using cyanogen bromide, sodium azide and 50% solution of hydroxylamine as raw materials (Scheme 1). As depicted, the cyanogen azide intermediate generated by the reaction of cyanogen bromide and sodium azide, could be followed by azido-oxime-cyclizationreaction with excess of aqueous hydroxylamine yielding 1-hydroxy-5-aminotetrazole hydroxylammonium salt. Whereafter, the —NH2group of above hydroxylammonium salt was completed oxidation-coupling by reaction of potassium permanganate at the present of potassium hydroxide, yielding 1,1′-dioxide-5,5′-azotetrazole dipotassium salt.
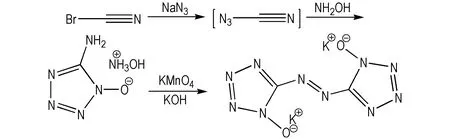
Scheme 1 Synthesis of 1,1′-dioxide-5,5′-azotetrazole dipotassium salt
2.2.1 1-Hydroxy-5-aminotetrazole Hydroxylammonium Salt
Cyanogen bromide (6.36 g, 60 mmol) was dissolved inacetonitrile(300 mL). The solution was cooled to 0 ℃ and the sodium azide (4.20 g, 64.5 mmol) was added. After stirring for 4 h in ice bath, the suspension was filtered and washed with MeCN (30 mL). Then, the filtrate was cooled to -20 ℃ and a 50% solution of hydroxylamine (13.2 g, 200 mmol) in MeCN (60 mL) was added dropwise in order to keep the temperature under 0 ℃. The resulting suspension was stirred for 1 h at room temperature and then filtered. The white solid was washed with Et2O (50 mL) and dired in air to afford pure compound (6.27 g, 78% yield).
IR(KBr,ν/cm-1):2469(m), 1744(w), 1645(w), 1561(m), 1536(m), 1451(w), 1308(m), 1266(w), 1258(w), 1204(w), 1165(w), 1143(w), 1125(w), 1102(m), 1006(m), 860(s), 805(w);
1H NMR(DMSO-d6)δ: 8.73, 8.25.
13C NMR(DMSO-d6)δ: 148.65.
Anal.Calcd. for CH6N6O2(%): C 8.96, H 4.51, N 62.67; Found: C 9.43, H 4.36, N 62.41.
2.2.2 1,1′-Dioxide-5,5′-azotetrazole Dipotassium Salt
1-Hydroxy-5-aminotetrazole hydroxylammonium salt (2.69 g, 20 mmol) was dissolved in water (80 mL) and 2 M KOH (15 mL) was added. The solution was heated to 75 ℃ and a solution of KMnO4(3.178 g, 20 mmol) in hot water (70-80 ℃) was added dropwise. After stirring for 1 h at 75 ℃, MeOH (25 mL) was added in one portion and the mixture was stirred for a further 30 min at 75 ℃. The resulting suspension was filtered and the filtrate was concentrated until the red solid product started to precipitate. The solid-liquid mixture was left to precipitate completely by adding of EtOH. The precipitate was filtered and washed with EtOH and Et2O to afford orange-red solid (2.25 g, 82% yield).
IR(KBr,ν/cm-1): 1744(w), 1418(m), 1367(w), 1247(w), 1219(m), 1135(m), 1062(w), 785(vs), 715(w).
13C NMR(DMSO-d6)δ: 168.71.
Anal.Calcd. for K2C2N10O2(%): C 8.76, N 51.07; Found: C 9.01, N 51.59.
3 Results and Discussions
3.1 Synthesis
According to the literature[17], 1-hydroxy-5-aminotetrazole could be readily prepared by the reaction of the cyanogen azide intermediate (generated by the reaction of cyanogen bromide and sodium azide) with excess of hydroxylammonium, as depicted in Scheme 1. It is found obviously that if one equivalent of hydroxylamine is applied in the reaction, 1-hydroxy-5-aminotetrazole hydroxylammonium salt in a low yield (<10%) from the MeCN solution. The yield can be increased about 90% when using two equivalent of hydroxylammonium. Because the reaction is quite exothermic which leads to a temperature rise of about 20 ℃ and an yellow unstable by-product always formed during the reaction, it was useful to cool the reaction medium down to -20 ℃ before the addition of hydroxylammonium as well as the speed of droprise should be controlled slowly. The above mentioned yellow unstable by-product always decomposes to gaseous products slowly while the quantity of white main-product increased at room temperature. This unstable by-product is probably another addition product because hydroxylammonium can also attack through the oxygen atom resulting correspondingN-amino tetrazole derivative (Scheme 2).
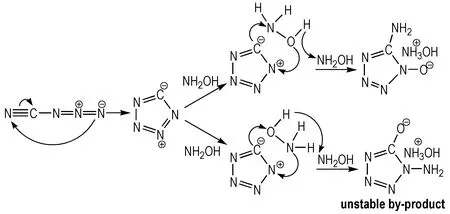
Scheme 2 Reaction mechanism of cyanogen zaide and hydroxylammonium
Because hydroxylamine can be destroyed in hot basic medium, the 1,1′-dioxide-5,5′-azotetrazole dipotassium salt can be prepared directly by coupling reaction of —NH2group in 1-hydroxy-5-aminotetrazole hydroxylammonium salt using excess of potassium permanganate as oxidizer. The any excess potassium permanganate in oxidation state is destoryed by the addition of MeOH after complete coupling. Therefore, the pure 1,1′-dioxide-5,5′-azotetrazole dipotassium salt can be isolated after the removal of dioxide manganese.
3.2 Thermal Performance
The DSC and TG-DTG analysis was carried out at a heating rate of 5 ℃·min-1from 50 ℃ to 500 ℃ under N2atmosphere. The DSC curve showed in Fig. 1 reveals that the 1,1′-dioxide-5,5′-azotetrazole dipotassium salt is thermally stable up to 270 ℃ and has no melting point, but exists two thermal decompositon peaks at 271.0 ℃ and 328.0 ℃, respectively. The TG-DTG curves showed in Fig.2 exhibits that 1,1′-dioxide-5,5′-azotetrazole dipotassium salt possess two-stage decomposition process with a mass loss of 28.60% before 300 ℃ in the first stage decomposition and a total mass of 37.5% before 320 ℃ in the second stage decomposition.
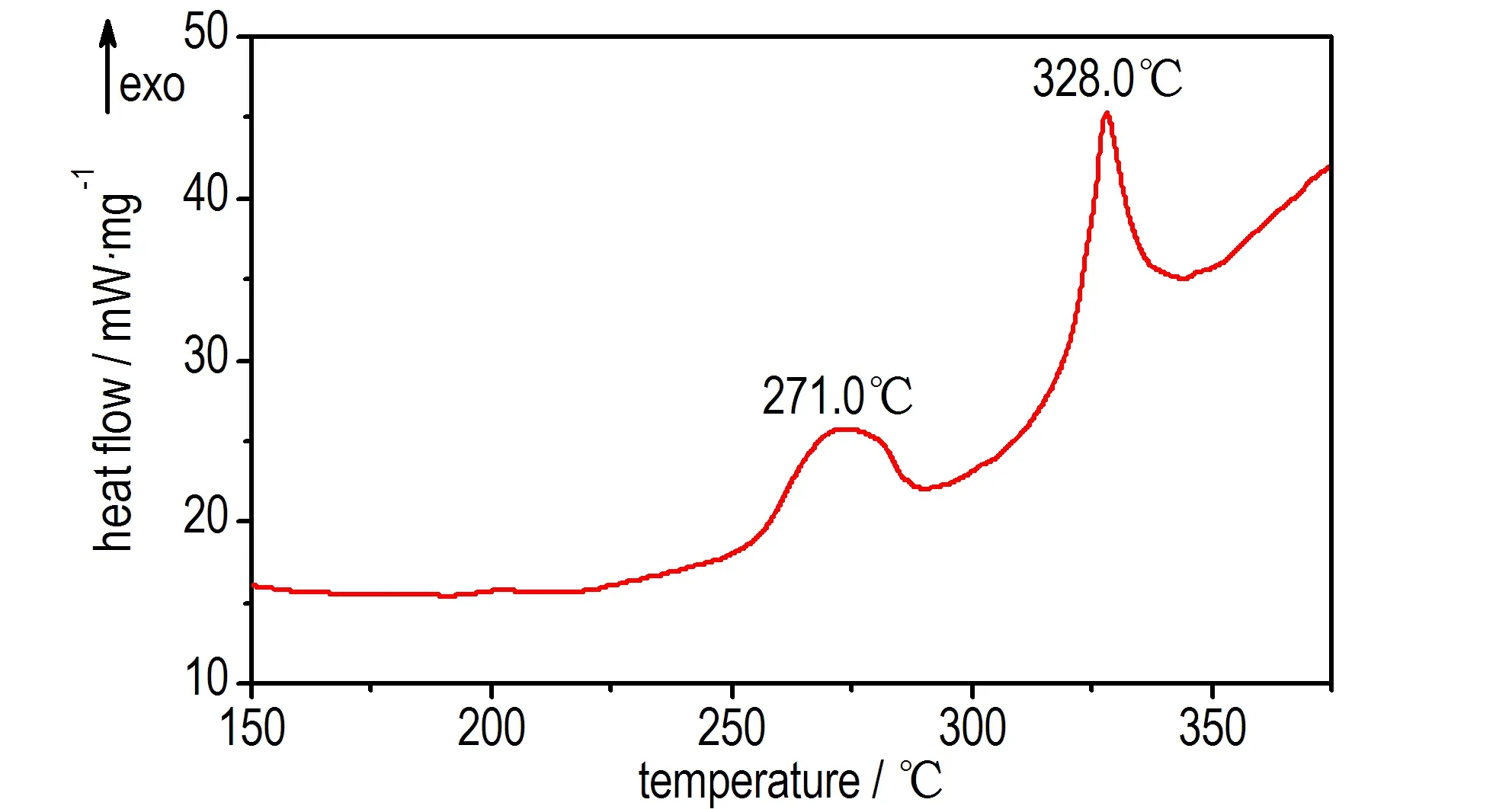
Fig.1 DSC curve of 1,1′-dioxide-5,5′-azotetrazole dipotassium salt
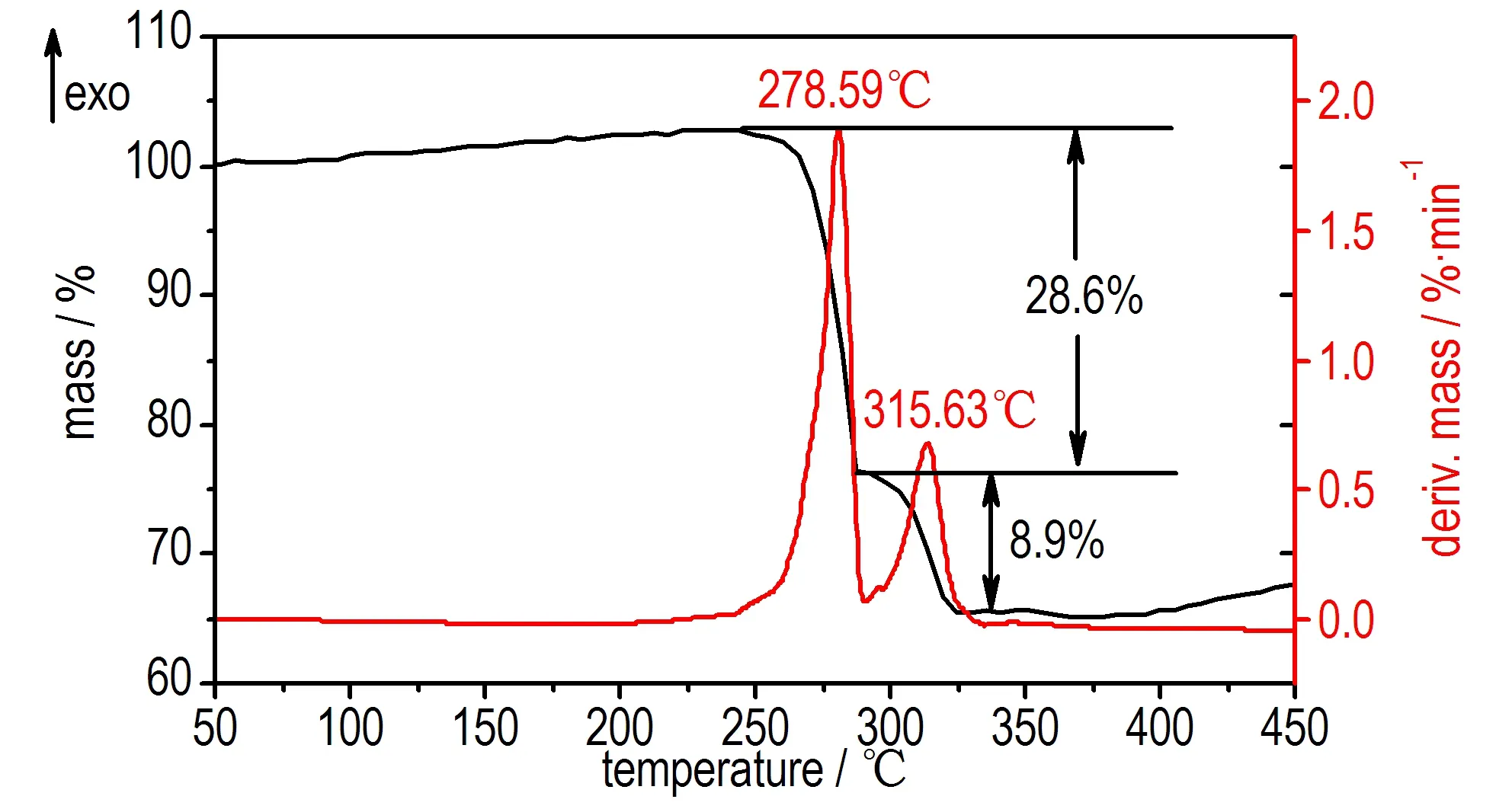
Fig.2 TG-DTG curve of1,1′-dioxide-5,5′-azotetrazole dipotassium salt
4 Conclusions
1,1′-Dioxide-5,5′-azotetrazole dipotassium salt was synthesized using cyanogen bromide, sodium azide and 50% solution of hydroxylamine as raw materials via two-step reactions of azido-oximes-cyclization and oxidation-coupling. DSC and TG-DTG curves indicates that 1,1′-dioxide-5,5′-azotetrazole dipotassium salt possess two thermal decompositon peaks at 271.0 ℃ and 328.0 ℃ under heating condition, respectively, while possess two-stage decomposition process with a mass loss of 28.60% before 300 ℃ in the first stage decomposition and a total mass of 37.5% before 320 ℃ in the second stage decomposition.
[1] Zhang M X, Eaton P E, Gilardi R. Hepta and octanitrocubanes[J].AngewChemIntEd, 2000,39(2): 401-404.
[2] Tao G H, Twamley B, Shreeve J M. A thermally stable nitrogen-rich energetic material—3,4,5-triamino-1-tetra-olyl-1,2,4-triazole (TATT)[J].JMaterChem, 2009,19: 5850-5854.
[3] Chavez D E, Hiskey M A, Naud D L, et al. Synthesis of an energetic nitrate ester[J].AngewChemIntEd, 2008, 47(43): 8307-8309.
[4] Bushuyev O S, Brown P, Maiti A, et al. Ionic polymers as a new structural motif for high-energy-density materials[J].JAmChemSoc, 2012, 134(3):1422-1425.
[5] Huynh M H V, Hiskey M A, Chavez D E, et al. Synthesis, characterization, and energetic properties of diazido heteroaromatic high-nitrogen C—N compound[J].JAmChemSoc, 2005, 127(36): 12537-12543.
[6] Chavez D E, Hiskey M A, Gilardi R D. Novel high-nitrogen materials based on nitroguanyl-substituted tetrazines[J].OrgLett, 2004, 6(17): 2889-2891.
[7] Li Y C, Qi C, Li S H, et al. 1,1′-Azobis-1,2,3-triazole: ahigh-nitrogen compound with stable N8 structure and photochromism[J].JAmChemSoc, 2010,132(35):12172-12173.
[8] Klapötke T M, Piercey D G. 1,1′-Azobis(tetrazole): ahighly energetic nitrogen-rich compound with a N10 chain[J].InorgChem, 2011, 50(7): 2732-2734.
[9] Klapötke T M, Stierstorfer J. The CN7— anion[J].JAmChemSoc, 2009, 131(3): 1122-1134.
[10] Carlqvist P, Ostmark H, Brinck T. The stability of arylpentazoles[J].JPhysChemA, 2004, 108(36): 7463-7467.
[11] Göbel M, Karaghiosoff K, Klapötke T M, et al. Nitrotetrazolate-2N-oxides and the strategy of N-oxide introduction[J].JAmChemSoc, 2010, 132(48): 17216-17226.
[12] Gao H, Shreeve J M. Azole-based energetic salts[J].ChemRev, 2011, 111(11): 7377-7436.
[13] Thottempudi V and Shreeve J M. Synthesis and promising properties of a new family of high-density energetic salts of 5-nitro-3-trinitromethyl-1H-1,2,4-triazole and 5,5′-bis(trinitromethyl)-3,3′-azo-1H-1,2,4-triazole[J].JAmChemSoc, 2011, 133: 19982-19992.
[14] Klapötke T M, Piercey D G, Stierstorfer J. The taming of CN7—: the azidotetrazolate 2-oxide anion[J].ChemEurJ, 2011,17(46): 13068-13077.
[15] Churakov A M, Tartakovsky V A. Progress in 1,2,4-tetrazine chemistry[J].ChemRev, 2004, 104(5): 2601-2616.
[16] Fischer N, Fischer D, Klapötke T M, et al. Pushing the limits of energetic materials-the synthesis and characterization of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate[J].JMaterChem, 2012, 22: 20418-20422.
[17] Fischer D, Klapötke T M, Piercey D G, et al. Synthesis of 5-aminotetrazole-1N-oxide and its azoderivative: akey step in the development of new energetic materials[J].ChemEurJ, 2013, 19: 4602-4613.
杂志排行
含能材料的其它文章
- Crystal Structure and Thermal Behavior of Potassium Dinitromethane
- Coatings of Activated Metal Hydride and Application in the Fuel-rich Propellant
- Synthesis and Properties of 5-(3-Amino-1,2,5-oxadiazol-4-yl)tetrazol-1-ol and Its Ammonium and Hydroxylammonium Salts
- The Tensile Properties and Creep Performance of a Long-term Thermally Aged Plastic Bonded Explosive
- Energies of Combustion and Specific Heat Capacities of Diaminofurazan, Dinitrofurazan and Diaminoazofurazan
- Thermal Behaviors of 1-Amino-2-nitroguanidine