Effect of Ammonia on the Performance of Catalysts for Selective Hydrogenation of 1-Methylnaphthalene
2015-06-22GePanzhuRenLiangGaoXiaodongLiDadong
Ge Panzhu; Ren Liang; Gao Xiaodong; Li Dadong
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Effect of Ammonia on the Performance of Catalysts for Selective Hydrogenation of 1-Methylnaphthalene
Ge Panzhu; Ren Liang; Gao Xiaodong; Li Dadong
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
The effect of ammonia on the catalytic performance for 1-methylnaphthalene (1-MN) selective hydrogenation saturation was studied with Co-Mo/γ-Al2O3, Ni-W/γ-Al2O3, Ni-Mo/γ-Al2O3, and Ni-Mo-W/γ-Al2O3catalysts. The results indicated that Ni-Mo-W/γ-Al2O3catalyst exhibited the best performance for saturation of 1-MN. The introduction of NH3remarkably inhibited the hydrogenation of 1-MN in the dynamic control area, but it had no effect in the thermodynamic control area. Besides, the mono-aromatics selectivity on the Ni-Mo-W and Ni-Mo catalysts was enhanced. However, it had little effect on the Ni-W and Co-Mo catalysts.
1-methylnaphthalene (1-MN); selectivity; hydrogenation catalyst; hydrogenation saturation
1 Introduction
With the increasingly stringent environmental regulations, clean gasoline and diesel production has become a growing concern[1]. Light cycle oil (LCO) of FCC unit features high contents of sulfur, nitrogen and aromatic hydrocarbons, low cetane number and poor stability[2]. Therefore, it is difficult to meet the clean diesel quality standards. At present, the processes of hydro-upgrading to produce high cetane number diesel need more severe operating conditions and higher hydrogen consumption. If the polyaromatic hydrocarbons (PAHs) in LCO can be selectively saturated into mono-aromatic hydrocarbons (MAHs), the MAHs could be cracked into high octane number components by hydrocracking or catalytic cracking to obtain higher economic value[3-4]. No matter LCO hydrocracking process or LCO hydrotreating combined with FCC process, the selective hydrogenation of PAHs is the key reaction.
Although, many studies concerning the effect of NH3on inhibiting the hydrotreatment of various heterocyclic and aromatic compounds have been reported[5-7], the effect of NH3on the selective hydrogenation saturation of aromatics is rarely studied. By using the NiMo/Al2O3catalyst, Lee and co-workers[8-9]found that NH3had little influence on the HDN of quinoline, but it significantly inhibited the deep hydrogenation of poly-aromatics (naphthalene and phenanthrene). Whether NH3in the presence of different catalysts can inhibit the deep hydrogenation of polyaromatics to improve mono-aromatic selectivity remains unclear. Therefore, the intention of this paper is to investigate the effect of NH3on the performance of different catalysts for selective hydrogenation saturation of 1-MN.
2 Experimental
2.1 Feedstock and catalysts
Feedstock: 1-MN was dissolved in cyclohexane to obtain a solution of A containing 10% of 1-MN. 1-MN and secbutylamine were dissolved in cyclohexane to obtain a solution of B containing 10% of 1-MN and 500 μg/g of nitrogen element. Sec-butylamine could rapidly generate NH3under reaction conditions. The effect of NH3on the performance of four different catalysts for selective hydrogenation of 1-MN was investigated with solution B.
Catalysts: The catalysts were the commercially available NiMoW/γ-Al2O3, NiMo/γ-Al2O3, NiW/γ-Al2O3and CoMo/γ-Al2O3catalysts developed by the Research Institute of Petroleum Processing (RIPP). The main composition and physical properties of catalysts are shown in Table 1.
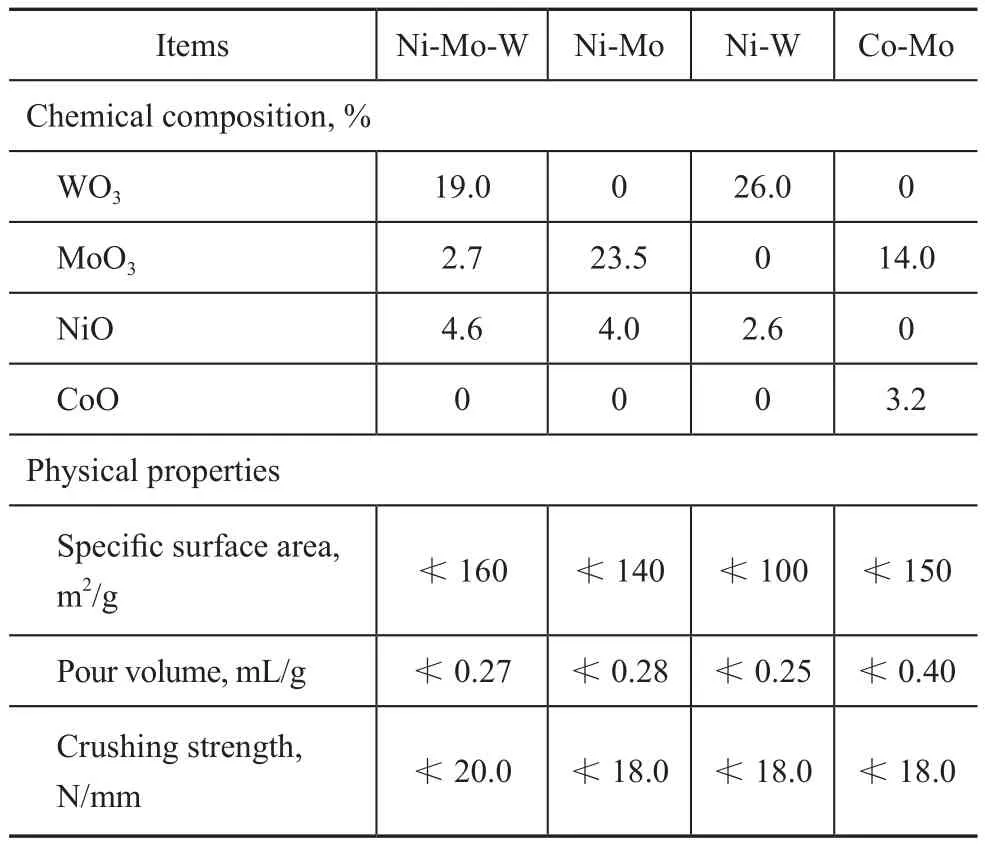
Table 1 Composition and physical properties of catalysts
2.2 Experiment on 1-MN hydrogenation
The effect of NH3on the performance of four different catalysts for selective hydrogenation saturation of 1-MN was studied by using a high pressure hydrogenation micro-reactor. The catalyst samples were crushed and sieved to a size range of 40—60 mesh. 0.5 g of catalyst was diluted with 1 g of the similar-sized inert SiC and packed into the reactor tube. The catalyst sample was presulfided at 300 ℃ for 4 h with 5 m% of CS2solution at a rate of 0.3 mL/min. The hydrogen flow rate was 300 mL/min under a total reaction pressure of 4.0 MPa. Then the liquid flow was switched to raw materials at a rate of 0.2 mL/min at a reaction temperature of 250—380 ℃and under a total reaction pressure of 4.0 MPa.
2.3 Analysis
Liquid samples of the reactor effluents were collected from a low-pressure phase separator until a steady level of activity was reached. Hydrocarbon components were analyzed on a type 7890 gas chromatograph manufactured by the Agilent Company of USA. The conversion of 1-MN (x1-MN) and the selective hydrogenation saturation of single rings and double rings (s1-ringand s2-ring) are calculated according to Equations (1)— (3), respectively.

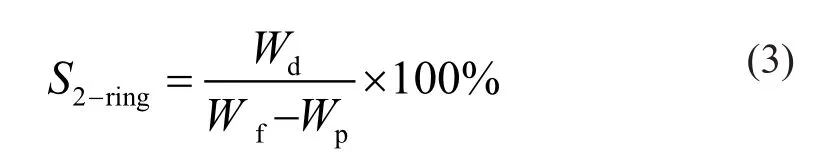
In Equations (1)—(3), wfand wprepresent the mass fraction of 1-MN in the raw material and product, respectively; wtand wdrepresent the mass fraction of tetralin and decalin, respectively.
Many studies show that the aromatics hydrogenation over metal sulfide catalysts (Co-Mo, Ni-Mo, and Ni-W) approximates to the first order reaction[10]. So the pseudofirst-order kinetic equation was used to analyze the reaction rate constant and the apparent activation energy. The pseudo-first-order kinetic equation is shown as follows.
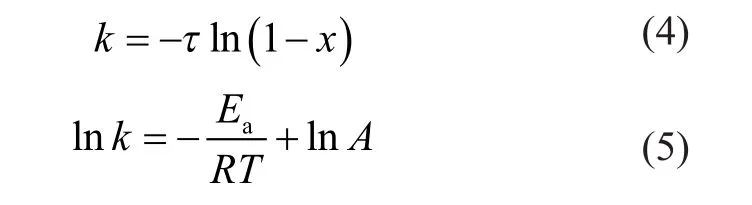
In Equations (4) and (5), τ represents the reciprocal of contact time, s-1; x represents the conversion rate of 1-MN hydrogenation; k represents the reaction rate constant of 1-MN hydrogenation; Earepresents the apparent activation energy of 1-MN hydrogenation, kJ/mol; R represents the gas constant; T represents the reaction temperature, K; and A represents the pre-exponential factor.
3 Influence of Internal and External Diffusion
3.1 Influence of internal diffusion
In order to make the dynamic data reliable, the study must rule out the influence of internal and external diffusion. Changing the catalyst granularity is an effective method to test the influence of internal diffusion.
By using the solution A as feed in the presence of catalyst samples with particle size in the range of 30—50 mesh and 50—70 mesh, respectively, the contrast experiment was carried out at a temperature of 350 ℃. Meanwhile, 0.5 g of catalyst was diluted with 1 g of the similar-sized inert SiC and packed into the reactor tube. The test results are shown in Table 2. It can be seen that the conversion rate of 1-MN was almost consistent with each other. Therefore, the influence of internal diffusion did not exist in the catalyst sample with a particle size of 30—70 mesh. So the catalyst with a particle size of 20—40 mesh was used in this paper.
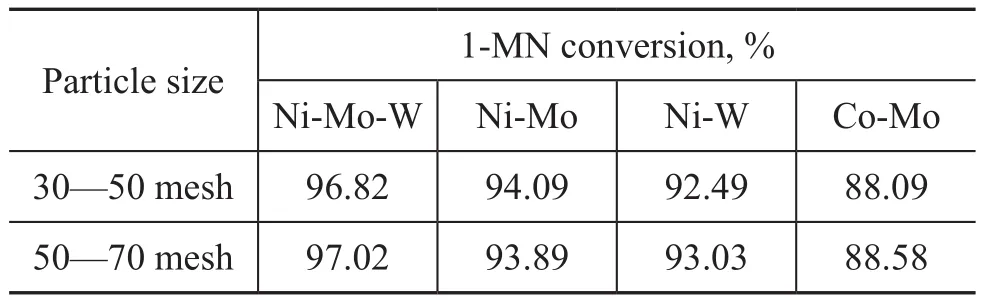
Table 2 Results of influence of internal diffusion
3.2 Influence of external diffusion
Changing the quantity of catalyst loading is an effective method to test the influence of internal diffusion, which means changing the linear speed of the material flow within the reactor.
By using the solution A as feed in the presence of catalyst with a particle size of 40—60 mesh, the contrast experiment was carried out with a loading of 0.3 g, 0.5 g and 0.8 g of catalyst samples diluted with SiC, respectively. The reaction temperature was 350 ℃ with other operating conditions remaining unchanged.
The test results are shown in Table 3. It can be seen that the conversion rate of 1-MN was almost the same and the influence of external diffusion did not exist when the catalyst loading was within 0.3—0.8 g. Thus 0.5 g of catalyst diluted with 1.0 g of the similar-sized inert SiC was used in this paper.
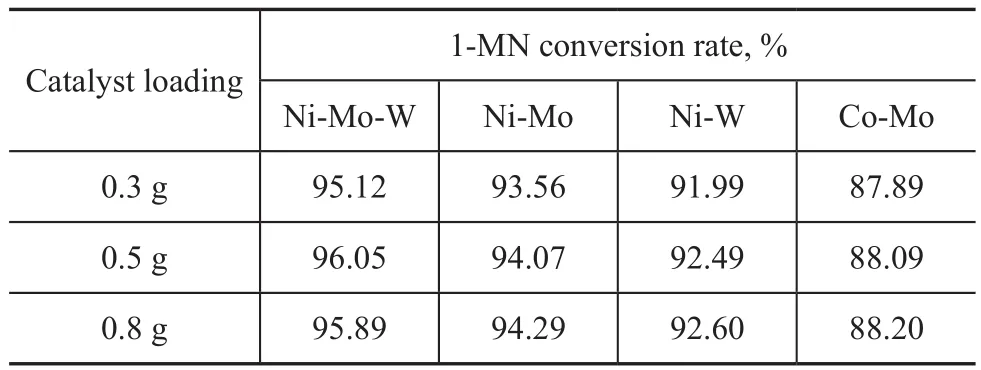
Table 3 Results of influence of external diffusion
4 Results and Discussion
4.1 Effect of NH3on 1-MN hydrogenation
The reaction network of naphthalene hydrogenation includes parallel and sequential reactions such as ring hydrogenation, ring opening, isomerization and demethylation[11-13]. The primitive reactions of 1-MN hydrogenation are complicated and the rate constants of reaction are related to the catalyst type[14-15]. Besides, whether NH3will influence the hydrogenation of 1-MN over different catalysts is also worth inspecting. Therefore, the experiments were conducted on the four catalysts under the conditions of either without addition of NH3or with addition of NH3. The change in 1-MN hydrogenation conversion rate with the reaction temperature is shown in Figure 1(a) and 1(b). The pseudo-first-order rate constants and apparent activation energy of 1-MN hydrogenation on different catalysts are summarized in Table 4.
According to Figure 1(a), when the reaction temperature was lower than 350 ℃, the catalyst activity of 1-MN saturation decreased in the following order: Ni-Mo-W>Ni-Mo>Ni-W>Co-Mo. The higher the activity of aromatic saturation was, the easier it would be for the 1-MN to get into the thermodynamic control area. When the reaction temperature was higher than 350 ℃, the activity order for four types of catalysts was basically the same. This phenomenon might be attributed to the fact that the catalyst might only change the reaction rate, but could not alter the equilibrium of the reaction when 1-MN saturation reaction reached the thermodynamic equilibrium at highertemperature[16]. According to the data listed in Table 4, when the reaction atmosphere did not contain NH3, the apparent activation energy of 1-MN hydrogenation on the Ni-W catalyst was lower than that of Co-Mo catalyst, so the aromatics saturation activity of the Ni-W catalyst was obviously higher than that of the Co-Mo catalyst.
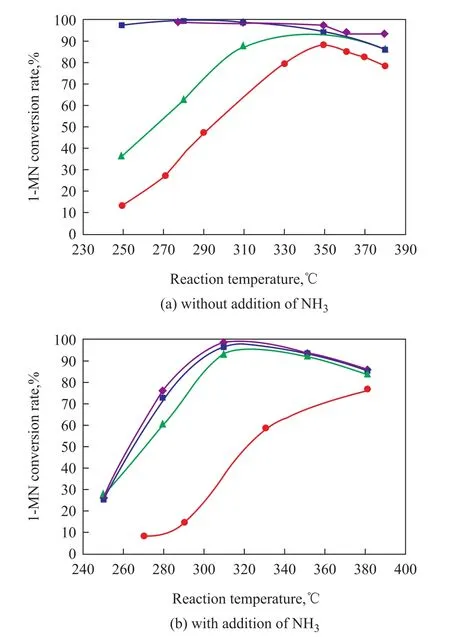
Figure 1 The effect of NH3on the 1-MN hydrogenation conversion rate using different catalysts■—Ni-Mo;●—Co-Mo;▲—Ni-W;◆—Ni-Mo-W
Upon comparing Figure 1(b) with Figure 1(a), the influence of NH3on the four catalysts was different. The introduction of NH3decreased the aromatics saturation activity of the Co-Mo catalyst, which also could be evidenced by the data in Table 4. The calculated values of the rate constants are consistent with data obtained by Lee, et al[8]. As for the more active Ni-Mo-W, Ni-Mo and Ni-W type catalysts, the aromatics saturation activity was basically the same. Besides, the introduction of NH3inhibited the transformation of 1-MN in the dynamic control area at the reaction temperature in the range of between 280 ℃and 310 ℃. However, when the reaction temperature was higher than 310 ℃, the aromatics saturation activity was basically the same in the thermodynamic control area. This suggested that NH3showed a significant inhibitory effect in the kinetic control area for the reaction involving 1-MN and tetralin, but it had no impact on the thermodynamic control area for the reaction involving 1-MN and tetralin[17]. The results can be further confirmed by the data listed in Table 4. After the introduction of NH3, the apparent activation energy for 1-MN hydrogenation decreased in the following order: Ni-Mo-W>Ni-Mo>Ni-W>Co-Mo, denoting that the higher the activity of the catalyst, the more obvious the inhibition effect of NH3would be.
According to Figure 1(b), the effect of NH3on inhibition of the Co-Mo type catalyst activity gradually slowed down with the increase of reaction temperature. This can be explained from two aspects. On the one hand, upon comparing with the influence of NH3, the limits of thermodynamic equilibrium between 1-MN and tetralin at higher temperature might be more obvious. On the other hand, a higher temperature improved the conversion frequency of reactants on the surface active centers of catalyst, which could weaken the competitive adsorption effect of NH3.
4.2 Effect of NH3on the products of 1-MN hydrogenation saturation
The products of naphthalene hydrogenation reaction are more complicated. Generally, the reaction network of naphthalene includes two parallel paths: one is that naphthalene is hydrogenated into tetralin, then the isomerization or ring opening reaction of tetralin occurs; the other is that naphthalene is hydrogenated into tetralin, tetralin is further hydrogenated into cis/trans-decalin, and then the isomerization or ring opening reaction of decalin occurs[12]. The reaction network of naphthalene hydrogenation is shown in Figure 2[11]. Tetralin and cis/trans-decalin are the main products of naphthalene hydrogenation.
By using the four types of catalysts, the 1-MN hydrogenation saturation reaction was carried out at temperatures ranging from 250 ℃ to 380 ℃ and under a hydrogen partial pressure of 4.0 MPa. The main products of 1-MN hydrogenation included tetralin, decalin and a small amount of indan and alkyl benzene obtained from isomerization and ring opening reactions. However, as regards the hydrogenation catalysts, the main products included tetralinNH3, which denoted that NH3increased the selectivity of tetralin in the temperature range of 310—380 ℃, but it showed little inhibitory effect on the selectivity of tetralin on the Ni-W catalysts. Besides, the introduction of NH3showed an obvious inhibitory effect on the generation of tetralin and had no effect on the selectivity of tetralin for the less active Co-Mo catalyst.

Table 4 Effect of NH3on 1-MN hydrogenation reaction rate constant and activation energy
4.3 Effect of NH3on the selectivity of 1-MN hydrogenation saturation
Under the same saturation rate of 1-MN, the effects of and decalin. The amount of indan and alkyl benzene was relatively small. Thus the trends of tetralin and decalin formation related with reaction temperature on the four types of catalysts are shown in Figure 3. Solid lines represent the case without NH3in the atmosphere, and dotted lines represent the case with the introduction of NH3into the atmosphere.
It can be seen from Figure 3 that, with regard to the Ni-Mo-W and Ni-Mo catalysts, the amount of tetralin increased and decalin decreased after the introduction of NH3on the selectivity of intermediate products from 1-MN hydrogenation saturation on the four types of catalysts were analyzed, with the results presented in Figure 4. As shown in Figure 4, with regard to the Ni-Mo-W and Ni-Mo catalysts, the presence of NH3in the reaction atmosphere was beneficial to improving the selectivity of the intermediate products of tetralin under the same saturation rate of 1-MN, which might be caused by the different competitive adsorption of NH3, tetralin and 1-MN on the catalyst surface. Some research results have shownthat the adsorption effect of the three substances decreases in the following order: NH3> naphthalene > tetralin[18]. Therefore, NH3is more likely to occupy the active centers of catalyst than tetralin because of its strong adsorption ability. On the other hand, NH3can easily inhibit the dissociative adsorption of hydrogen molecules, which would reduce the concentration of tetralin and hydrogen molecules on the catalyst surface and further inhibit the hydrogenation of tetralin[7,19-21]. Because naphthalene has strong adsorption activity and NH3has less inhibitory effect on the naphthalene compared with tetralin, the introduction of NH3improves the selectivity of tetralin on the Ni-Mo-W and Ni-Mo catalysts.
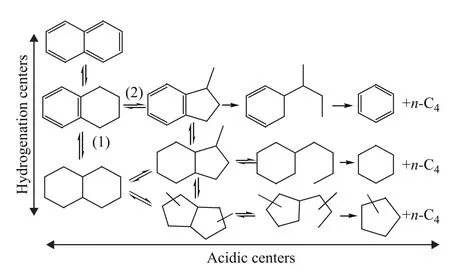
Figure 2 Reaction network of naphthalene hydrogenation
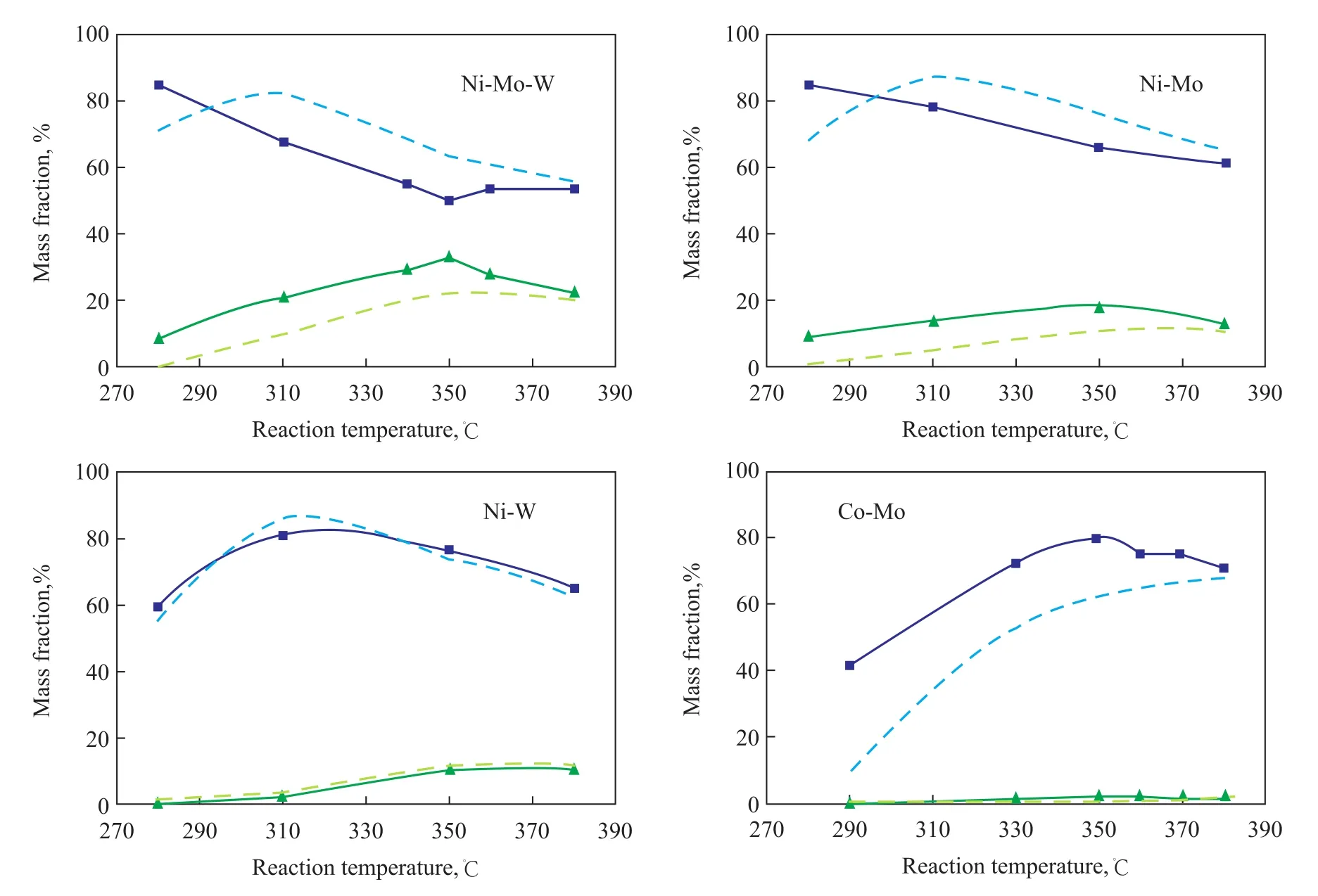
Figure 3 Trends of tetralin and decalin formation on the four types of catalysts■—Tetralin;▲—Decalin;---Tetralin (NH3);---Decalin (NH3)
Nevertheless, the introduction of NH3has no effect on the selectivity of tetralin on the Ni-W and Co-Mo catalysts, as shown in Figure 4. These results might be ascribed to the lower aromatics saturation activity of Ni-W and Co-Mo catalysts. The products originating from deep saturation of decalin were so insignificant that the effect of NH3on the selectivity of tetralin was not obvious. This phenomenon may also indicate that NH3has no influence on the selectivity of tetralin on the Ni-W and Co-Mo catalysts.

Figure 4 Effect of NH3on the selectivity of 1-MN hydrogenation saturation■—No added NH3;◆—With addition of NH3
5 Conclusions
1) The catalyst activity for 1-MN saturation decreased in the following order: Ni-Mo-W>Ni-Mo>Ni-W>Co-Mo, and the higher the activity of catalyst for aromatics saturation, the easier the 1-MN feedstock could get into the thermodynamic control area.
2) In the dynamic control area of aromatics hydrogenation saturation, the introduction of NH3could significantly inhibit the transformation of 1-MN. However, in the thermodynamic control area, the introduction of NH3had no effect on the conversion rate of 1-MN.
3) The introduction of NH3could significantly increase the selectivity of mono-aromatics on the Ni-Mo-W and Ni-Mo catalysts, but it had little effect on the Ni-W and Co-Mo catalysts.
Acknowledgement:This project is financially supported by the SINOPEC.
[1] Li Dadong. Pertroleum refining technologies and catalysis in the 21stcentury[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2005, 21(3): 17-25 (in Chinese)
[2] Huang Xinlu, Zeng Ronghui. A discussion on FCC diesel processing schemes[J]. Sino-global Energy, 2012, 17: 75-82 (in Chinese)
[3] Mao Anguo,Gong Jianhong. Molecular-based study on FCC LCO to light aromatics[J]. Petroleum Processing and Petrochemicals, 2014, 45(7): 1-6 (in Chinese)
[4] Huang Xinlu. Technology for producing light aromatics from heavy aromatics[J]. Chemical Industry and Engineering Process, 2013, 32(9): 2263-2266 (in Chinese)
[5] Jiang Donghong, Shi Yulin, Hu Zhihai. Influence of H2S and NH3on the activity of aromatics hydrogenation over sulfidized hydrotreating catalyst[J]. Petroleum Processing and Petrochemicals, 2006, 37(4): 25-27 (in Chinese)
[6] Chadwick D, Oen A, Siewe C. Influence of water and ammonia on hydrotreating catalysts and activity for tetralin hydrogenation [J]. Catalysis Today, 1996, 29: 229-233
[7] Nat P J, Schoonhoven J, Plantenga F L. Mild and conventional hydrocracking: process conditions, products and catalysts[J]. Studies in Surface Science and Catalysis, 1989, 53: 399-415
[8] Lee C M, Satterfield C N. Effect of ammonia on the hydrogenation of naphthalene or butylbenzene during the hydrodenitrogenation of quinoline[J]. Energy & Fuels, 1992, 6(3): 315-317
[9] Lee C M, Satterfield C N. Effect of ammonia on the hydrogenation of phenanthrene during the hydrodenitrogenation of quinoline[J]. Energy & Fuels, 1993, 7(6): 978-980
当解释变量为外部评价主体时,ICR=1,否则,ICR=0。从模型3能够发现,ω2反映会计稳健性和正股票收益率之间的关系,ω4反映会计稳健性和负股票收益率之间的关系,若ω4显著为正,则表明“坏消息”比“好消息”能更快速反映会计稳健性。在本文中可以解释为若在进行内部控制评价时,公司出具的内部控制评价报告具有真实性,则公司的会计稳健性将显著高于内部控制评价报告不真实的公司的会计稳健性。
[10] Sapre A V, Gates B C. Hydrogenation of Aromatic Hydrocarbons Catalyzed by Sutfided Co-Mo/γ-Al2O3. Reactivities and Reaction Networks[J]. Industrial & Engineering Chemistry Process Design and Development, 1981, 20(1): 68-73
[11] Liu Yunqi, Li Wangliang, Liu Chunying, et al. Hydrogenation of naphthalene on HY/MCM-41/γ-Al2O3supported sulfided Ni-Mo-P catalyst[J]. Chinese Journal of Catalysis, 2004, 25(7): 537-541 (in Chinese)
[12] Ren Xiaoqian, Yu Xizhi, Li Kai, et al. Reactivity of saturated hydrogenation of naphthalene over the commercial NiW/Al2O3catalyst at high reaction temperature[J]. Chemical Engineering, 2007, 35(3): 30-34 (in Chinese)
[13] Liu Lihua, Liu Shuqun. Ni2P-MoS2/γ-Al2O3catalyst for deep hydrodesulfurization via the hydrogenation reaction pathway[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(4): 12-18
[14] Korret S C, Klein M T. Polynuclear aromatic hydrocarbons hydrogenation. 1. Experimental reaction pathways and kinetics [J]. Ind Eng Chem Res, 1995, 34: 101-117
[16] Wang Zhenglie, Zhou Yaping. Physical Chemistry (Part II) [M]. Beijing: China Higher Education Press, 2001: 271-272 (in Chinese)
[17] Li Hongbao, Huang Weiguo, Kang Xiaohong, et al. Effect of nitrogen compounds on aromatics hydrogenation over Ni-W hydrotreating catalysts with various supports[J]. Petroleum Processing and Petrochemicals, 2006, 37(10): 27-32 (in Chinese)
[18] Lau Y K, Saluja P P S, Kebarle P, et al. Gas-phase basicities of N-methyl substituted 1,8-diaminonaphthalenes and related compounds[J]. Journal of the American Chemical Society, 1978, 100(23): 7328-7333
[19] Blanchin S, Galtier P, Kasztelan S, et al. Kinetic modeling of the effect of H2S and of NH3on toluene hydrogenation in the presence of a NiMo/Al2O3hydrotreating catalyst. Discrimination between homolytic and heterolytic models[J]. The Journal of Physical Chemistry A, 2001, 105(48): 10860-10866
[20] Shao Zhicai, Zhao Xinqiang, Liu Tao, et al. Commercial application of the second generation RHT catalysts for hydroprocessing the residue with low sulfur and high nitrogen contents[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(1): 1-7
[21] Shao Zhicai, Liu Tao, Dai Lishun, et al. Effect of H2S and NH3on heteroatomic removal by hydrogenation[J]. Petroleum Processing and Petrochemicals, 2014, 45(3): 30-34 (in Chinese)
date: 2015-09-14; Accepted date: 2015-11-04.
Prof. Gao Xiaodong, Telephone: +86-10- 82369334; E-mail: gaoxd.ripp@sinopec.com.
猜你喜欢
杂志排行
中国炼油与石油化工的其它文章
- Computational Fluid Dynamics Simulation of Liquid-Phase FCC Diesel Hydrotreating in Tubular Reactor
- Hydrothermal Liquefaction of Wheat Straw in Sub-critical Water/Ethanol with Ionic Liquid for Bio-oil Production
- Microbial Characterization of Denitrifying Sulfide Removal Sludge Using High-Throughput Amplicon Sequencing Method
- Promotional Effect of CoO(OH) on Selective Hydrogenation of Maleic Anhydride to γ-Butyrolactone over Supported Ruthenium Catalyst
- Quantitative Analysis Using Fourier Transform Ion Cyclotron Resonance Mass Spectrometry and Correlation between Mass Spectrometry Data and Sulfur Content of Crude Oils
- Design and Control of Self-Heat Recuperative Distillation Process for Separation of Close-Boiling Mixtures: n-Butanol and iso-Butanol
