Model of limestone calcination/sulfation under oxy-fuel fluidized bed combustion
2015-05-08WangChunboLiuHongcaiChenLiang
Wang Chunbo Liu Hongcai Chen Liang
(School of Energy Power and Mechanical Engineering, North China Electric Power University, Baoding 071003, China)
Model of limestone calcination/sulfation under oxy-fuel fluidized bed combustion
Wang Chunbo Liu Hongcai Chen Liang
(School of Energy Power and Mechanical Engineering, North China Electric Power University, Baoding 071003, China)
The characteristics of the simultaneous calcination/sulfation of limestone under oxy-fuel fluidized bed combustion were studied and compared with those of the sulfation of precalcined CaO. During the calcination stage, SO2can react with product CaO and slow down the CaCO3decomposition rate by the covering effect of the CaSO4product. The sulfation rate of simultaneous calcination/sulfation is slower than that of precalcined CaO, but with a long enough sulfation time, the calcium conversion of simultaneous calcination/sulfation is higher than that of the precalcined CaO. A grain-micrograin model is established to describe the simultaneous calcination, sintering and sulfation of limestone. The grain-micrograin model can reflect the true reaction process of the calcination and sulfation of limestone in oxy-fuel fluidized bed combustion.
oxy-fuel; limestone; simultaneous calcination/sulfation; grain-micrograin model
Under the oxy-fuel fluidized bed combustion conditions, CO2concentration in flue gas can be enriched up to 80% (air-dry basis) or even higher because of recycle gases. Limestone is usually used for controlling SOxemission. Furthermore, it can greatly decrease NOxemissions because there is no N2in the combustion atmosphere. Therefore, oxy-fuel combustion is one of promising new technologies that can integrally control the discharge of pollutants by coal combustion[1-3]. The relationship of limestone decomposition temperature and CO2partial pressure is[4]
(1)
Under the condition of 80%CO2, the temperature of the limestone calcined to CaO is 885 ℃, and at the operating temperature (850 ℃) of the conventional CFBB, the sulfation of sorbent is called direct sulfation:
(2)
However, when the fuel is petroleum coke or anthracite, the temperature of operation in the furnace is over 900 ℃ and the sulfation occurring in this way is called indirect sulfation:
CaCO3→CaO+CO2
(3)
(4)
The common experimental method in previous studies concerning reactions (2) and (4) is: First, limestone is calcined to form CaO, and then the sulfation characteristics of CaO are investigated[5-8]. However, under the oxy-fuel CFBB combustion conditions, the CO2concentration in flue gas is high, which will delay the decomposition of limestone, and the limestone will be calcined and sulphated simultaneously.
In the past decades, many models were established to simulate the process of calcination and sulfation of limestone. The random pore model was established and used for the reaction of CaO and SO2by Bhatia et al.[9-11]and the grain model was founded by Szekely et al[12]. Hartman et al.[13]modified the pore structure. However, most of the sulfation models treat the calcination and sulfation of limestone separately, which is suitable for the sulfation of limestone under the furnace injection conditions due to the high reaction temperature and small particle size causing an instant calcination[14-16]. Yet, they are not appropriate for sulfation in the CFBB when the calcination and sulfation occur simultaneously over a relatively long duration. A combined calcination and sulfation model for reaction of SO2and CaCO3was built[17]to study the calcination and sulfation of limestone integrally under furnace injection conditions, but the applicability of this model to the oxy-fuel CFBB is not clear.
In this work, a grain-micrograin model was used to simulate the process of simultaneous calcination, sintering and sulfation of limestone under the oxy-fuel CFBB conditions. The calcination and sulfation experiments were carried out in a constant temperature tube furnace which can record sample weight change continuously to study the simultaneous calcination and sulfation reactions and verify the model.
1 Model for Simultaneous Calcination and Sulfation
The grain-micrograin model for the simultaneous calcination and sulfation of limestone is presented and the physical schematic is shown in Fig.1. The original limestone particles are assumed to be nonporous as shown in Fig.1(a). CaCO3grains are calcined from outside to inside and CaO micrograins generate around the uncalcined CaCO3grain. Meanwhile, CaO micrograins are sintered and sulfated. The SO2from bulk gas and CO2generated in the decomposition reaction diffuse inside and outside respectively through the pores among CaO micrograins. The CaSO4product layers was generated around the CaO micrograins, as shown in Fig.1(b). The heat transfer resistance was ignored in the particle, so the temperature of the particle is equal to the ambient temperature, and gas diffusion resistance from the surface of the particle to bulk gas is also ignored. Pseudo-steady-state approximation is adopted here. According to the analysis above, the grain-micrograin model is described as follows.

Fig.1 Grain-micrograin model. (a) The structure of a lomestone grain; (b) A sulfated CaO micrograin
The calcination reaction rate is assumed to be proportional to the reaction rate constant and reaction surface area, and inversely proportional to the CO2pressure of the reaction area. So the calcination rate of limestone is
(5)
wherekcis the reaction rate constant andpeis the CO2equilibrium partial pressure of limestone. CO2generated in the decomposition reaction diffuses outside through the gaps between the CaO micrograins, which can be described by
(6)
with the initial conditionst=0,p=0; and the boundary conditions
whereDeis the effective diffusion coefficient of CO2and it is primarily controlled by the Kundsen diffusion,
(7)
andDkcis the Kundsen diffusion coefficient
(8)
SO2from flue gas diffuses inside through the gaps between the CaO micrograins, which can be described as
(9)
with boundary conditions
rSO2is the sulfation reaction rate, andDseis the effective diffusion coefficient of SO2in the CaO particles,
(10)
in whichDksis the Kundsen diffusion coefficient,
(11)
andrkis the average pore radius between the CaO micrograins
(12)
whereεiis the local porosity of the particle andSiis the local specific surface area. Due to the sintering and sulfation, the specific surface area and porosity of porous CaO decrease quickly. The sintering rate of CaO is heavily influenced by the specific surface area, which has been investigated by many researchers, and the sintering rate meets the two order dynamic rules[18-19]. So, the change of specific surface area caused by sintering can be calculated by
(13)
whereSais the asymptotic specific surface area with the value of about 5 m2/g[19], andKshis the sintering rate constant.
Integrating Eq.(13), we can obtain
(14)
The porosity is related to the specific surface area as
(15)
The local porosityεSdecreases along with the sulfation reaction because the molar volume of CaSO4is larger than that of CaO. According to Hartman et al[13], the porosity change can be described as
εS=ε0-(Z-1)(1-ε0)χS
(16)
So the local porosity of CaO can be described as
(17)
withε0=0.54 andS0=104 m2/g according to the investigation of Borgwardt[20].Zis the molar volume ratio of CaSO4to CaO, andχSis the local sulfation conversion of CaO.
The local specific surface area of CaO particles can be calculated by
(18)
The calcination conversion of CaCO3to CaO is calculated by
(19)
in whichrpis the initial radius of CaCO3grain.
The sulfation rate of CaO can be calculated by
(20)
with the initial conditionst=0,r1=r2=r0.
The sulfation rate of per unit grain volume based on the state ion diffusion obtained by Mahuli[17]is
(21)
The sulfation rate of sintered CaO equals the diffusion rate of state ion through the CaSO4layer according to Lindner[21], so
(22)
whereL=r1-r2;Dionis the state ion diffusion coefficient;CCis the state ion density on the reaction surface equal to the molar density of CaO; and Savis the average cross-sectional area of state ion diffusion.
Combining Eqs.(21) and (22), we obtain the sulfation rate controlled by intrinsic kinetics and product layer state ion diffusion as
(23)
For CaO grains at the same area,
rSO2=rCaO
(24)
Combining Eqs.(20), (23) and (24), we obtain
(25)
The local sulfation conversion conversionχSis
(26)
and the grain size changes along with the sulfation reaction
(27)
The Ca conversion to CaSO4can be obtained by integrating local conversion conversion of all layers, so
(28)
The relative quality change at the simultaneous calcination and sulfation is
(29)
2 Experiment Facility and Procedure
The isothermal thermo-gravimetric experiment system to investigate simultaneous calcination and sulfation of limestone at constant temperature is shown in Fig.2. The tube furnace is 40 mm in diameter and 800 mm in length. The temperature in the furnace was monitored by an automatic controller with a range of 20 to 1 200 ℃. The change of sample weights was monitored by the computer constantly and the precision of the weight sensor was 0.1 mg. Simulated flue gas consists of 75%CO2, 5%O2, 0.2%SO2and N2as balance. In all tests, the flow rate of the gas mixtures was maintained at 1 200 mL/min. This flow rate is selected as it has been verified that at this flow rate, the mass transfer is not the limiting factor for the reactions. Previous work[22-23]operated on this device has verified that it has sufficient accuracy.
Shandian limestone was used in the experiment. The X-ray fluorescence (XRF) analysis result of limestone is given in Tab.1. A known amount of limestone sample (about 80 mg) was loaded into a quartz boat (100 mm in length, 10 mm in width and 10 mm in depth). In order to be close to the working conditions of industry as much as possible, the temperature was raised to the desired level and maintained for 60 min, and then the quartz boat (with limestone) was sent into the furnace quickly.

Tab.1 Main component of limestone %
The calcium conversion of sample (after limestone decomposes into CaO completely) is calculated by
(30)

Fig.2 Experimental system
whereXis the conversion of calcium;mtis the mass at the timet;m0is the original sample mass of CaO formed by calcined limestone without SO2;miis the initial sample mass of limestone;Ais the mass fraction of the CaCO3in the original samples;wCaCO3,wCaSO4andwCaOare the molar mass of CaCO3, CaSO4and CaO, respectively.
The experiments of simultaneous calcination and sulfation (termed simultaneous calcination/sulfation) are carried out with 0.2%SO2; and are compared, experiments in which limestone calcined in pure N2to CaO and then sulfated (termed calcination-sulfation) with 0.2% SO2are also given.
3 Results and Discussion
The characteristics of simultaneous calcination/sulfation and calcination-sulfation along with the calculation result of the model is shown in Fig.3.
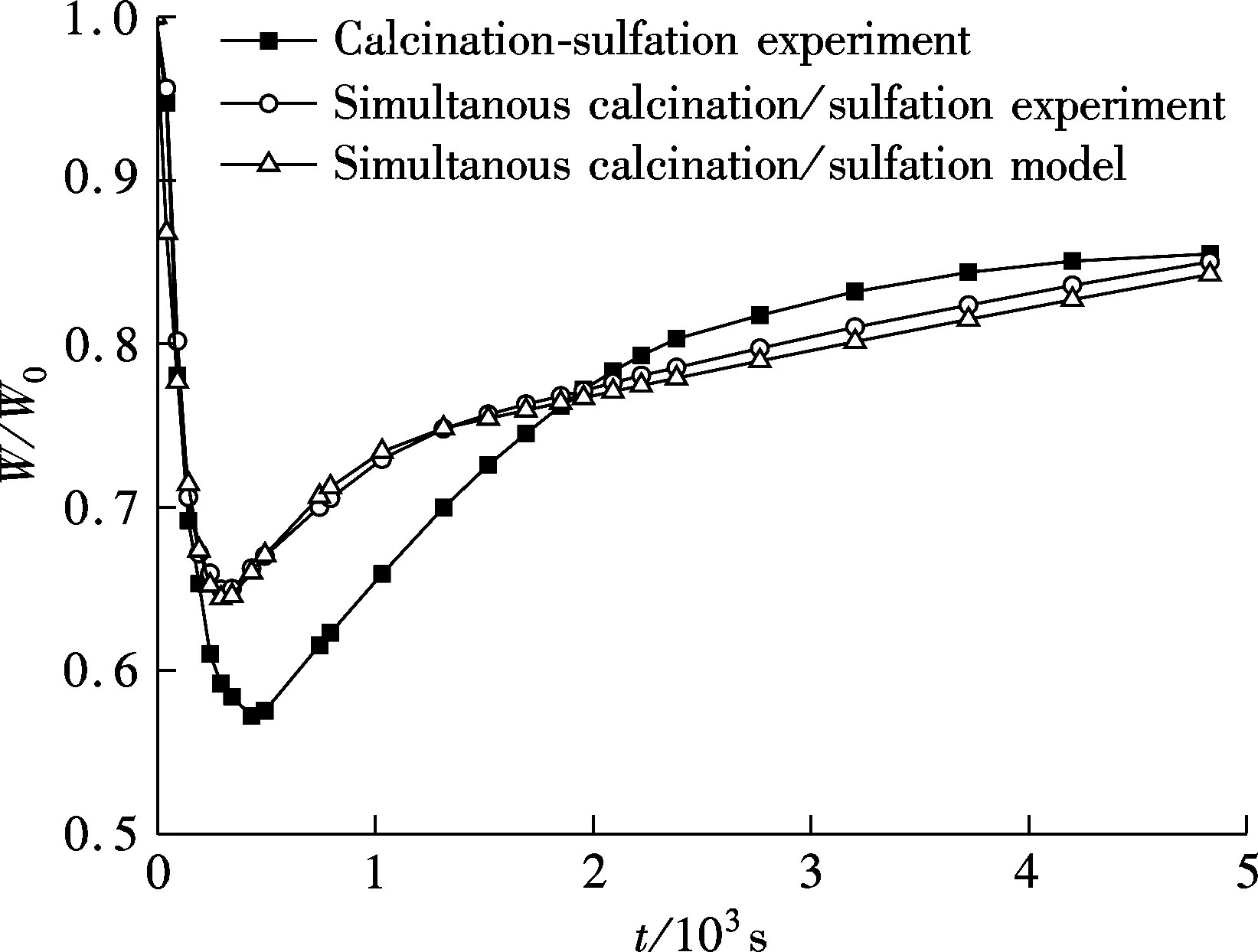
Fig.3 Tesing and model prediction(900 ℃, 150 to 250 μm, 0.2%SO2,5%O2, 75%CO2, N2 as balance)
Fig.3 shows the ratio of sample weightWto the initial weightW0in the process of experiment. First, a significant difference can be found when comparing the experimental data of calcination-sulfuration to those of the simultaneous calcination/sulfation reaction. Compared with calcination-sulfuration, the weight loss rate of simultaneous calcination/sulfation in the weight decline stage is slightly slower, but the lowest weight point is obviously higher. The cause is that the calcination and sulfation reaction occurs at the same time in the calcination stage of the simultaneous calcination/sulfation experiment, which, on the one hand, increases the sample weight due to the sulfation of CaO; and on the other hand, prevents the calcination of CaCO3by the product layer of CaSO4. What should be pointed out is that when calcination and sulfation occur simultaneously, the limestone loses weight due to calcination but gains weight by sulfation; which can lead to the result that the lowest weight point is not the point that limestone is calcined completely usually, but only a balance point between weight gain and loss.
The next significant difference obtained from Fig.3 is that during the period of weight rise, although there remains two reaction stages which include a fast reaction stage controlled by reaction kinetics and a slow reaction stage controlled by product layer diffusion for both of the two experimental conditions; the percentage of weight gain is relatively lower in the early stage and higher in the later stage for simultaneous calcination/sulfation experiments.
The calculating results of the grain-micrograin model established is consistant with the testing results and can be used to describe the simultaneous calcination and sulfation of limestone, as shown in Fig.3. Even if in pure N2, it needed about 500 s for the limestone particle of 150 to 250 μm to be calcined completely. Therefore, it is necessary to take the sulfation of CaO into account in this long calcination stage, because not only the calcination of CaCO3but also the sulfation of CaO are influenced by it. Data will not reflect reality if sulfation of the precalcined CaO is taken as the sulfation of limestone in an oxy-fuel CFBC. Simultaneous sulfation with calcination of limestone should not be ignored for both experiment and model derivation when studying sulfation phenomenon in oxy-fuel CFBB.
To calculate the sulfation ratio of limestone, it must be ensured that all CaCO3in the sample is calcined to CaO completely. To detect if there is CaCO3in the samples, several samples undergoing different reaction times after the lowest weight point are quickly cooled in N2to room temperature and grinded to less than 10 μm, and then calcined again at 900 ℃ in pure N2within sufficient time. If a sample does not lose weight in this process, it can be ensure that there is no CaCO3in the sample. Also, the test results show that the samples after 680 s do not lose mass anymore, which means that there is no CaCO3in these samples. So, the calcium conversion formula can be used to calculate the calcium conversion to CaSO4, as shown in Fig.4.
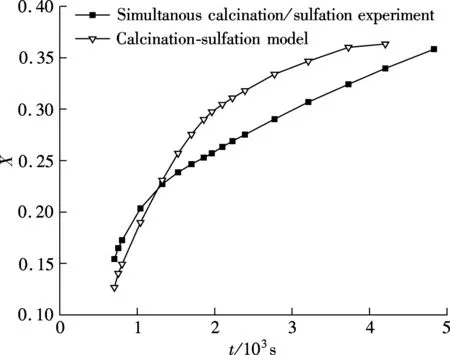
Fig.4 Conversion for simultaneous calcinations/sulfation and calcinations-sulfation of limestone
From Fig.4, it can be shown that the sulfation rate of calcination-sulfation experiment was faster at first but declined quickly when compared with simultaneous calcinations/sulfation. Although the calcium conversion of calcination-sulfation is higher after the cross point in Fig.4, its conversion rate slowed down finally, so if with a longer reaction time, the conversion of simultaneous calcinations/sulfation should exceed that of calcinations-sulfation.
Perhaps one contrast test is not sufficient to illustrate the problems, so several other contrast tests and model calculations are carried out, and Fig.5 and Fig 6 show two of them.
Fig.5 is the result of experiment and model calculation for a particle size of 75 to 97 μm, and Fig.6 is for a temperature of 950 ℃ compared with Fig.4. It can be found that a similar phenomenon occurs for different experimental temperatures and particle sizes, which illustrates that the differences between simultaneous calcination/sulfation and calcination-sulfation conditions are not an accidental phenomenon. The grain-micrograin model established in this work can also describe the true process of simultaneous calcination/sulfation under different experimental conditions.
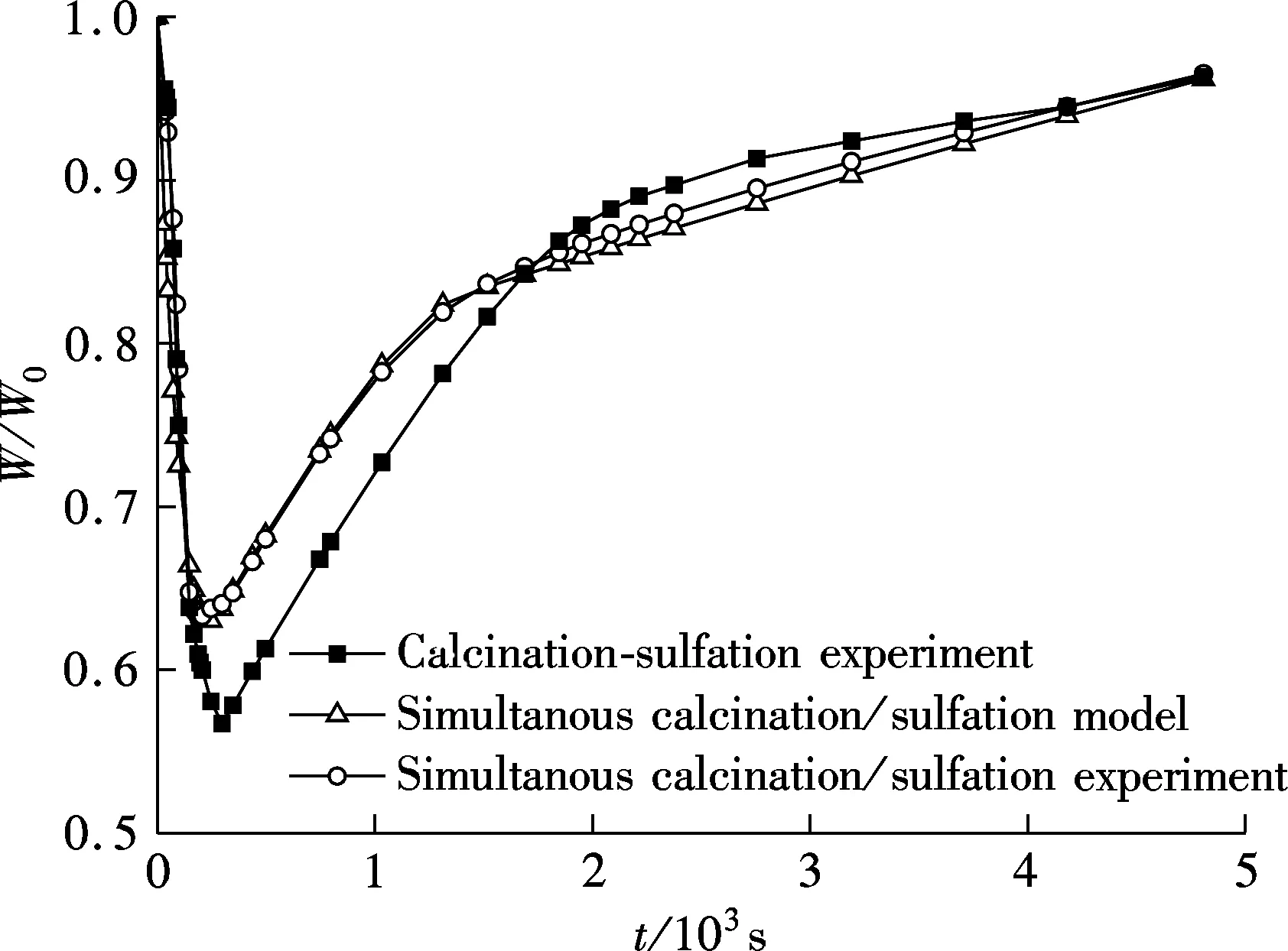
Fig.5 Tesing and model prediction (900 ℃, 75 to 97 μm, 0.2%SO2, 5%O2, 75%CO2, N2 as balance)

Fig.6 Tesing and model prediction (950 ℃, 150 to 250 μm, 0.2%SO2, 5%O2, 75%CO2, N2 as balance)
4 Conclusion
The simultaneous calcination/sulfation of limestone under oxy-fuel fluidized bed combustion conditions is different from the sulfation of CaO. Also, during the calcination stage, SO2will slow down the CaCO3decomposition rate via the covering effect of the CaSO4product. The sulfation rate of simultaneous calcination/sulfation declines slower than that of precalcined CaO, and with a long enough sulfation time, the calcium conversion of simultaneous calcination/sulfation is higher than that of precalcined CaO. The grain-micrograin model combining simultaneous calcination, sintering and sulfation can reflect the true process of the calcination and sulfation of limestone under an oxy-fuel fluidized bed combustion. When studying the calcination and sulfation of limestone under oxy-fuel CFBB conditions, it is necessary to pay attention to the calcination and sulfation of limestone at the same time.
[1]Liu H, Okazaki K. Simultaneous easy CO2recovery and drastic reduction and NOxin O2/CO2coal combustion with heat recirculation[J].Fuel, 2003, 82(11): 1427-1436.
[2]Liu Hao, Ren Ruiqi, Huang Yongjun, et al. Reduction and emission of NO in oxy-fuel system[J].JournalofChemicalIndustryandEngineering, 2011, 62(2): 495-501. (in Chinese)
[3]Duan Lunbo, Zhou Wu, Qu Chengrui, et al. SO2emission from a coal-fired circulating fluidized bed combustor under O2/CO2atmosphere[J].JournalofEngineeringThermophysics, 2012, 33(1): 151-154.
[4]Baker E H. The calcium oxide-carbon dioxide system in the pressure range 1-300 atmopheres[J/OL].JournaloftheChemicalSociety(Resumed), 1962:464-470.http://pubs.rsc.org/en/content/articlepdf/1962/jr/jr9620000464.
[5]Wang W Y, Bjerle I. Modeling of high-temperature desulfurization by Ca-based sorbents[J].ChemicalEngineeringScience, 1998, 53(11): 1973-1989.
[6]Wang C, Jia L, Tan Y, et al. The effect of water on the sulfation of limestone[J].Fuel, 2010, 89(9): 2628-2632.
[7]Stewart M C, Manovic V, Anthony E J, et al. Enhancement of indirect sulfation of limestone by steam addition[J].EnvironmentalScienceandTechnology, 2010, 44(22): 8781-8786.
[8] García-Labiano F, Rufas A, de Diego L F, et al. Calcium-based sorbents behaviour during sulfation at oxy-fuel fluidised bed combustion conditions[J].Fuel, 2011, 90(10): 3100-3108.
[9]Bhatia S K, Perlmutter D D. A random pore model for fluid-solid reactions: Ⅰ. Isothermal, kinetic control[J].AIChEJournal, 1980,26(3):379-386.
[10]Bhatia S K, Perlmutter D D. A random pore model for fluid-solid reactions: Ⅱ. Diffusion and transport effects[J].AIChEJournal, 1981,27(2):247-254.
[11]Bhatia S K, Perlmutter D D. The effect of pore structure on fluid-solid reactions: application to the SO2-lime reaction[J].AIChEJournal1981, 27(2):226-234.
[12]Szekely J, Evans J W. A structural model for gas-solid reactions with a moving boundary[J].ChemicalEngineeringScience, 1970, 25(6):1091-1107.
[13]Hartman M, Coughlin R W. Reaction of sulfur dioxide with limestone and the grain model[J].AIChEJournal, 1976, 22(3):490-498.
[14]Silcox G D, Kramlich J C. A mathematical model for the flash calcination of dispersed CaCO3and Ca(OH)2particles [J].IndustrialEngineeringChemistryResearch,1989, 28(2):155-160.
[15]Milne C R, Silcox G D, Pershing D W, et al. High-temperature, short-time sulfation of calcium-based sorbents. 1. Theoretical sulfation model[J].IndustrialEngineeringChemistryResearch, 1990, 29(11): 2192-2201.
[16]Milne C R, Silcox G D, Pershing D W, et al. High-temperature, short-time sulfation of calcium-based sorbents.2. Experimental data and theoretical model predictions[J].IndustrialEngineeringChemistryResearch,1990, 29(11): 2201-2214.
[17]Mahuli S K, Agnihotri R, Jadhav R, et al. Combined calcination, sintering and Sulfation model for CaCO3SO2reaction[J].AIChEJournal, 1999, 45(2): 367-382.
[18]Ghosh-Dastidar A, Mahuli S, Agnihotri R, et al. Ultrafast calcination and sintering of Ca(OH)2powder:experimental and modeling[J].ChemicalEngineeringScience, 1995, 50(13): 2029-2040.
[19]Agnew J, Hampartsoumian E, Jones J M, et al. The simultaneous calcination and sintering of calcium based sorbents under a combustion atmosphere[J].Fuel, 2000, 79(12): 1515-1523.
[20]Borgwardt R H. Sintering of nascent calcium oxide[J].ChemicalEngineeringScience, 1989, 44(1): 53-60.
[21]Lindner B, Simonsson D. Comparison of structural models for gas-solid reactions in porous solids undergoing structural changes[J].ChemicalEngineeringScience, 1981, 36(9):1519-1527.
[22]Wang Chunbo, Zhang Yue, Jia Lufei, et al. Effect of water vapor on the pore structure and sulfation of CaO[J].Fuel, 2014, 130: 60-65.
[23]Wang Chunbo, Wang Jinxing, Lei Ming, et al. Investigations on combustion and NO emission characteristics of coal and biomass blends[J].Energy&Fuel, 2013, 27(10): 6185-6190.
富氧燃烧循环流化床中石灰石煅烧/硫化反应模型
王春波 刘洪才 陈 亮
(华北电力大学能源动力与机械工程学院,保定071003)
对富氧燃烧流化床下石灰石同时煅烧/硫化特性进行了研究,并与CaO硫化特性进行了比较.在石灰石煅烧阶段,CaO与SO2反应生成CaSO4产物层覆盖在未煅烧CaCO3的外层,降低了煅烧速率.同时煅烧/硫化过程中的硫化反应速率比CaO的硫化反应速率要缓慢,但是经过足够长的反应时间后,同时煅烧/硫化反应的钙转化率比CaO硫化反应的钙转化率要高.建立了一个包含石灰石同时煅烧、烧结和硫化反应的晶粒-微晶粒模型用于描述流化床内同时进行的石灰石煅烧、烧结和硫化过程,实验证明所建立的模型能够反映流化床富氧气氛中石灰石的真实煅烧/硫化过程.
富氧燃烧;石灰石;同时煅烧/硫化;晶粒-微晶粒模型
TK16
Foundation items:The National Natural Science Foundation of China (No.51276064), the Natural Science Foundation of Hebei Province (No.E2013502292).
:Wang Chunbo, Liu Hongcai, Chen Liang. Model of limestone calcination/sulfation under oxy-fuel fluidized bed combustion[J].Journal of Southeast University (English Edition),2015,31(2):238-243.
10.3969/j.issn.1003-7985.2015.02.014
10.3969/j.issn.1003-7985.2015.02.014
Received 2015-01-07.
Biography:Wang Chunbo (1973—), male, doctor, professor, hdwchb@126.com.
猜你喜欢
杂志排行
Journal of Southeast University(English Edition)的其它文章
- Adaptive modulation in MIMO optical wireless communication systems
- An improving energy efficiency cooperation algorithm based on Nash bargaining solution in selfish user cooperative networks
- Performance analysis of an O2/CO2 power plantbased on chemical looping air separation
- A novel carbon trap sampling systemfor coal-fired flue gas mercury measurement
- Applicability of Markov chain-based stochastic modelfor bubbling fluidized beds
- Composite bioabsorbable vascular stents via 3D bio-printingand electrospinning for treating stenotic vessels
