Dietary Leucine Requirement of Juvenile Japanese Seabass (Lateolabrax Japonicus)
2015-03-31LIYanCHENGZhenyanMAIKangsenandAIQinghui
LI Yan, CHENG Zhenyan, MAI Kangsen, and AI Qinghui
Dietary Leucine Requirement of Juvenile Japanese Seabass ()
LI Yan, CHENG Zhenyan, MAI Kangsen, and AI Qinghui*
,,266003,
A 56-day feeding trial was conducted to examine the dietary leucine requirement of juvenile Japanese seabass in seawater floating net cages (1.5m×1.5m×2.0m). Six isonitrogenous (crude protein 40%) and isoenergetic (gross energy 20kJg−1) diets were formulated to contain different concentrations of leucine (0.9%, 1.49%, 2.07%, 2.70%, 3.30% and 3.88% of dry matter). Crystalline L-amino acids were supplemented to simulate the whole body amino acid pattern of Japanese seabass except for leucine. Three groups (30 fish individuals each, 8.0g±0.20g in initial weight) were fed to apparent satiation at 5:00 and 17:30 every day. During the experimental period, the water temperature ranged from 26 to 32℃ and salinity from 26to 30, and the dissolved oxygen was maintained at 7mgL−1. The results showed that weight gain (), nitrogen retention (), feed efficiency () and protein efficiency ratio () were significantly increased when dietary leucine was increased from 0.90% to 2.70% of dry matter, and then declined.was the highest when fish were fed D4 containing 2.70% of leucine. No significant differences were observed in body composition among dietary treatments (0.05). Considering the change of, the optimum dietary leucine requirement of juvenile Japanese seabass was either 2.39% of dry matter or 5.68% of dietary protein.
growth; leucine; requirement; Japanese seabass
1 Introduction
Leucine is believed to be an indispensable amino acid in most aquatic species because its carbon skeleton cannot be synthesized. It functions as a major regulator in body metabolism at multiple levels (Balage., 2011). Usually, leucine can activate protein synthesis and decrease proteolysis, thus favoring a positive nitrogen balance (Dardevet., 2002; Crozier., 2005; Donato., 2007). Dietary leucine supplementation has been shown to reduce diet-induced obesity (Zhang., 2007; López., 2010; Vianna., 2012; Freudenberg., 2012; Eller., 2013) and inflammation in adipose tissue (Macotela., 2011; Toneto., 2012). In addition, it is also important to produce hemoglobin, maintainplasma glucose level and increase growth hormoneproduction. However, leucine deficiency may reduce growth and diet conversion (Wilson and Halver, 1986), and serve as an antagonist of valine and isoleucine when the proportion of these three amino acids in diets is mal-balanced (D’Mello, 2003). The antagonism has been found to depress the growth of chinook salmon (Chance., 1964), lake trout (Hughes., 1984) and rainbow trout (Yamamoto., 2004). Therefore, it is necessary to determine the appropriate dietary leucine content.
The amino acid requirement of cultured species can be decided with a well-developed method. Dietary protein level was fixed at the optimal crude protein, which was satisfied for the maximal growth of cultured species. The composition of amino acids in the diet was simulated by either whole chicken egg protein (Luo,2005; Khan and Abidi, 2007) or the whole body protein of cultured species (Alliot, 1974; Tibaldi and Tulli, 1999; Mai, 2006) excluding the target amino acid. Diets were made isonitrogenous and isoenergetic by adjusting the non-essential amino acids and carbohydrate (Millamena, 1997; Tibaldi and Tulli, 1999). Graded levels of a tested amino acid were supplemented to the basal diet, and the range of content covered the level of reference amino acids. Optimal requirement of target amino acid was estimated according to the growth performance of fish species (Tibaldi and Tulli, 1999; Khan and Abidi, 2007).
Japanese seabass is an economically important marine fish species and has been widely cultured in China. A few studies on its nutrient requirements have been conducted (Ai, 2004a, 2004b; Zhang,2005). Mai(2006) estimated that the dietary lysine requirement of juvenile Japanese seabass was 2.49% of dry diet (or 5.80% dietary protein) by broken-line analysis on the basis of(specific growth rate). In order to gain the maximal growth of the species, knowledge of the other indispensable amino acid requirements is very important. The purpose of the present study was to quantify the dietary leucine requirement of this species.
2 Materials and Methods
2.1 Experimental Diets
Six isonitrogenous (crude protein 40%) and isoenergetic (gross energy 20kJg−1) diets were formulated with six different levels of leucine ranging from 0 to 3.0% of dry diet with an increment of 0.6% (Table 1). Dietary leucine was quantitatively increased at the expense of glutamic acid. Glutamic acid was used to be blank, because it is non-essential and has little effect on the results of the experiment. L-crystalline amino acids mixture was used, so that the levels of all amino acids, except for leucine, would simulate the whole body amino acid pattern of Japanese seabass (initial body weight 8.0g±0.20g). The amino acid (AA) content of experimental diets is shown in Table 2. All the dietary AA contents were maintained nearly the same as the corresponding AA contents in 43% of whole body protein except for leucine and glutamic acid. The final level of leucine was 0.90%, 1.49%, 2.07%, 2.70%, 3.30% and 3.88% of dry weight, respectively, by adding crystalline leucine, which was determined by auto amino acids analyzer (Biochrom Ltd®, England). The range of dietary leucine content covered the leucine level (3.02%) in 43% crude protein from the whole body tissue of this species. The diets were marked as D1, D2, D3, D4, D5 and D6, respectively.
The ingredients were ground into fine powder through a 320μm mesh. The diets were prepared by thoroughly mixing the dry ingredients, blending with the oil and water, and then forcing the paste through a pelletizer (F-26 (II), South China University of Technology) to obtain pellets. The moist pellets were dried in an oven at 45℃ for 12h. The dry pellets were crushed and sieved to obtain suitable pellet sizes (1.5mm×2.0mm and 2.5mm×3.0mm), then sealed in bags and stored at −15℃ until used.
2.2 Experimental Procedure
Experimental fish were obtained from a commercial farm in Ningbo, China. Prior to the feeding trial, the fish were reared in floating sea cages (3.0m×3.0m×3.0m), and fed the control diet (D1) for two weeks to acclimate to the experimental diets and conditions. At the start of the experiment, the fish were fasted for 24h and weighed after being anesthetized with eugenol (1:10000) (Shanghai Reagent Corp, China). Juvenile fish in similar sizes were randomly assigned to 18 cages (1.5m×1.5m×2.0m), 30 each. Diets each were randomly assigned to three cages. To prevent the waste of dietary pellets, fish were slowly hand-fed in small batches at 05:00 and 17:30 every day till visual satiation of fish feeding behavior,, never coming up to water surface for feed. Feed consumption was recorded daily. Feeding trial lasted for 56 days. During the experimental period, the water temperature ranged from 26 to 32℃, and salinity from 26to 30. Photoperiod was about 14h−1d−1and dissolved oxygen was approximately 7mgL−1. At the end of the experiment, the fish were fasted for 24h and fish each cage were weighed and counted.
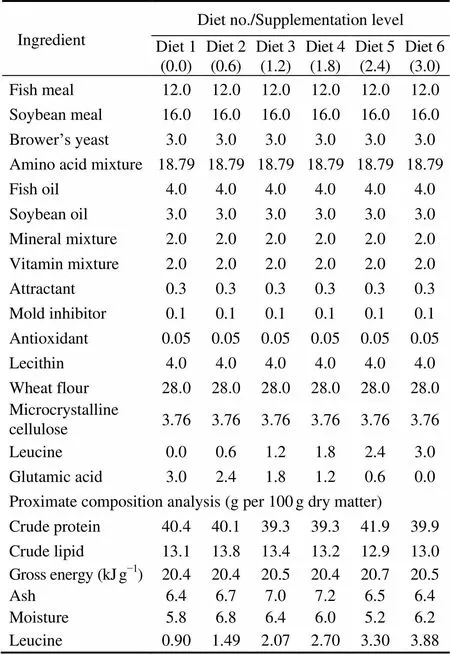
Table 1 Formulation and composition of the test diets used for the leucine requirement of Japanese seabass (g per 100g dry matter)
Notes: Fish meal (white fish meal): obtained from Hangzhou Wensli Biology Science and Technology Corporation (Zhejiang, China), crude protein 67.5% dry matter, crude lipid 7.8% dry matter; Soybean meal (de-hulled soybean meal): obtained from commercial market, crude protein 46.2% dry matter, crude lipid 1.7% dry matter; Beer yeast, crude protein 57.1% dry matter, crude lipid 3.5% dry matter; Wheat flour, crude protein 13.9% dry matter, crude lipid 1.9% dry matter. Amino acid mixture (gkg−1diet): arginine, 24.3; histidine, 4.8; isoleucine, 9.8; lysine, 23.5; methionine, 9.7; phenylalanine, 9.6; valine, 10.6; aspartic acid, 25.9; serine, 11.5; glycine, 16.6; alanine, 21.7; tyrosine, 8.1; glutamic acid, 40.7; threonine, 12.0. Mineral premix (mg or gkg−1diet): NaF, 2mg; KI, 0.8mg; CoCl2·6H2O (1%), 50mg; CuSO4·5H2O, 10mg; FeSO4·H2O, 80mg; ZnSO4·H2O, 50mg; MnSO4·H2O, 60mg; MgSO4·7H2O, 1200mg; Ca (H2PO4)2·H2O, 3000mg; NaCl, 100mg; Zoelite, 15.448g. Vitamin premix (mg or gkg−1diet): thiamin, 25mg; riboflavin, 45mg; pyridoxine- HCl, 20mg; vitamin B12, 0.1mg; vitamin K3, 10mg; inositol, 800mg; pantothenic acid, 60mg; niacin acid, 200mg; folic acid, 20mg; biotin, 1.20mg; retinol acetate, 32mg; cholecalciferol, 5mg; alpha-tocopherol, 120mg; ascorbic acid, 2000mg; choline chloride, 2000mg; ethoxyquin, 150mg; wheat middling, 14.52g. Attractant, glycine and betaine; Mold inhibitor, p-Aminobenzoic acid; Antioxidant: Ethoxyquin.

Table 2 Amino acid composition of ingredients and free amino acid supplementation (g per 100g dry matter)
Notes: FM, fish meal; SBM, soybean meal; WF, wheat flour; BY, brewer’s yeast. Change varied to 0–3.0%.
2.3 Measurement and Analysis
Ten fish per cage at the termination of the feeding trial were sampled and frozen (−20℃) for the analysis of proximate whole body composition. Proximate analysis on feedstuffs, diets and fish were performed according to the standard methods of AOAC (2003). Dry matter was achieved by drying in an oven set at 105℃ and the weight was constant (DHG-9140A, Shanghai). Crude protein (N×6.25) was measured by Kjeldahl method after acid digestion (FOSS Kjeltec 2300, Sweden). Lipid was assayed by ether extraction using Soxhlet (BUCHI B-811, Swist- zerland). Ash content was determined by incineration in a muffle furnace at 600℃ for 12h (SK-4-10, Shenyang, China). For amino acids analysis (except for methionine and cystine), the whole fish tissue samples were freeze- dried, and then hydrolyzed with 6molL−1HCl at 110℃ for 22h, followed by analyzing with an amino acids analyzer (Biochrom Ltd®, England). For methionine and cystine, the samples were oxidized with performic acid at −10℃ for 3h to obtain methionine sulfone and cysteic acid, and then freeze-dried twice with deionized water. The freeze-dried ingredients were hydrolyzed and analyzed by the reverse-phase high performance liquid chromatography (HPLC, HP1100, USA). The energy was measured with an adiabatic bomb calorimeter (PARR1281, USA).
2.4 Calculation and Statistical Analysis
The following variables were calculated:

,
,

Feed intake (%d−1),
wherefandiwere final and initial fish weights andis the experimental duration in days.
All data were subjected to variance and regression analysis if being appropriate using SPSS 16.0 for windows. Differences between the means were tested by Tukey’s multiple range test. The level of significance was chosen at<0.05. Comparing the coefficient of determination (2) of broken-line model
,
and second-order polynomial model
(Zeitoun, 1976).
The second-order polynomial model was chosen as the best fit model which gave maximum of2.
3 Results
3.1 Growth Performance
In the present study, the survival rate of Japanes seabass ranged from92.0% to 98.9%, and no significant difference in survival was found among diets (Table 3).significantly increased with the increase of dietary leucine concentration from 0.90% to 2.70%, and then significantly decreased when dietary leucine concentration increased from 2.70% to 3.88%.was the highest when fish fed D4 with 2.70% leucine content. There were no significant differences inof fish fed diets containing leucine over 0.90% of dry matter. However,s of fish fed D3 (2.07% leucine) and D4 (2.70% leucine) were significantly higher than those of fish fed D1 (0.90% leucine). The changing trends ofandwere similar with that of, significantly increasing with the increase of dietary leucine concentration from 0.90% to 2.07%, and then decreased when dietary leucine concentration increased from 2.07% to 3.88%.of fish fed D1 was the highest, which was significantly higher than that of fish fed D3 and D5. Considering the change of, the optimum dietary leucine requirement of juvenile Japanese seabass was 2.39% of dry diet (5.68% of dietary protein) (Fig.1), which was estimated through quadratic regression analysis.

Table 3 Effect of dietary leucine on the growth and survival of juvenile Japanese seabass (L. japonicus) fed experimental diets for 56 days†
Notes:†Value is the mean of three replicate groups (=3). Means with different letter in the same column differ significantly (<0.05);, weight gain;, specific growth ratio;, feed efficiency;, protein efficiency ratio;, feed intake; S.E.M., standard error of means; ANOVA, one-way analysis of variance.

Fig.1 Effect of dietary leucine on the weight gain (WG)of juvenile Japanese seabass (L. japonicus) fed experimental diets for 56 days.
3.2 Whole Body Composition
No significant difference was observed in the contents of body protein (16.2%–17.2%), lipid (5.8%–7.5%), moisture (71.8%–73.6%) and ash (4.5%–5.2%) among diets (0.05) (Table 4).
4 Discussion
In the present study,of Japanese seabass increased with the increase of dietary leucine content and then decreased when the content increased further. This result paralleled with that found in Indian major carp (Abidi and Khan, 2007). Our result indicated that leucine is essential for the growth of Japanese seabass; while dietary suboptimal or super optimal leucine can lead to a reduction in growth rate. The optimum dietary leucine requirement of juvenile Japanese seabass was 5.40%–5.98% of dietary protein within 95% confidence based on growth performance. The result was lower than that of blunt nose black bream (6.98%, Li, 1996), higher than those of other fishes,, 5.2% of Atlantic salmon (Rollin, 1999), 3.75%–3.92% of Indian major carp (Abidi and Khan, 2007), 4.30% of postlarval tiger shrimp (Millamena, 1999) and white sturgeon (Ng and Hung, 1995), 4.40% rainbow trout (Kaushik, 1998), 3.90 of Japanese flounder (Forster and Ogata, 1998) and chinook salmon (Chance, 1964), and 3.50% of channel catfish (Wilson, 1980). In this experiment, soybean meal was used as intact protein. Japanese seabass is carnivorous and cannot utilize soybean meal very well, which leads to a low feed intake and low digestibility of amino acids. This could also lead to a high leucine requirement. It is well known that the crystalline amino acids can be absorbed faster by fish than those of intact protein. In this present study, the fast absorption of crystalline amino acids leads to a poor utilization for protein synthesis. This may be one of possible reasons that leucine requirement of juvenile Japanese seabass was higher than those of some other fish species.
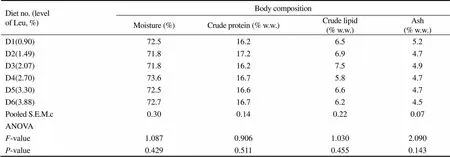
Table 4 Effect of dietary leucine on the body composition of juvenile Japanese seabass (L. japonicus) fed experimental diets for 56 days†
Notes:†Value is the mean of three replicate groups (=3); w.w., wet weight; S.E.M., standard error; ANOVA, one-way analysis of variance.
Amino acid balance in diets is necessary for the optimal growth of animals. Leucine deficiency can cause low diet intake, severe biochemical malfunction and growth retardation (de la Higuera, 2001). However, excessive dietary leucine may depress the growth response of Japanese seabass as was documented in Indian major carp (Abidi and Khan, 2007). The reason might be that the excessive leucine leads to accumulation and oxidation of ketones and other toxic metabolites, which adversely affect the growth of fish (Abidi and Khan, 2007). In addition, the antagonism of BCAA (Branched-chain amino acid) in different proportions can also inhibit growth, and it was severe in a high excessive BCAA (Yamamoto, 2004). In this experiment, when dietary leucine was not at the optimal level, an apparent antagonism among BCAA was observed, which depressed the growth of juvenile Japanese seabass. The antagonism has been found also in chinook salmon (Chance., 1964), lake trout (Hughes., 1984) and rainbow trout (Yamamoto., 2006).
Leucine is the only amino acid being able to reproduce the effect of a mixture of amino acids on muscle protein synthesis. Many previous studies found the ingestion of leucine was able to acutely increase the protein synthesis rate (Garlick, 2005; Kimball and Jefferson, 2006; Rieu, 2006). The body protein of Indian major carp was enhanced significantly with increasing dietary leucine concentrations up to 1.5%. However, beyond this level, a significant fall in body protein concentration was evident (Abidi and Khan, 2007). Choo. (1991) also proved that increasing dietary leucine did not increase the body protein of rainbow trout. In this study, there was no significant difference in protein, lipid and moisture contents. Probably, chronic supplementation of leucine or measurement of more sensitive parameters are necessary to detect the effect of supplemental leucine on nutrition status.
In a conclusion, leucine is essential for the growth of Japanese seabass, and fish can utilize crystalline leucine. On the basis of, the optimum dietary leucine requirement of juvenile Japanese seabass is 2.39% of dry diet (5.68% of dietary protein) as was estimated through second-order polynomial regression analysis.
Acknowledgements
The study was supported by the National Key Technologies R&D Program for the 15thFive-year Plan of China (Grant no. 2004BA526B-06) and Program for New Century Excellent Talents in University (NCET-07-0776). We thank Drs. C. X. Zhang, W. B. Zhang, X. J. Wang, H. M. Ma, J. K. Shentu and Q. Y. Duan for their assistances in the study.
Abidi,S. F., and Khan, M. A.,2007. Dietary leucine requirement of fingerling Indian major carp,(Hamilton)., 38: 478-486,DOI: 10.1111/j. 1365-2109.2007.01687.x.
Ai, Q. H., Mai, K. S., Li, H. T., Zhang, C. X., Zhang, L., Duan, Q. Y., Tan, B. P., Xu, W., Ma, H. M., Zhang, W. B., and Liufu, Z. G., 2004a. Effects of dietary protein to energy ratios on growth and body composition of juvenile Japanese seabass,.,230: 507-516, DOI: 10. 1016/j.aquaculture.2003.09.040.
Ai, Q. H., Mai, K. S., Zhang, C. X., Xu, W., Duan, Q. Y., Tan, B. P., and Liufu, Z. G., 2004b. Effects of dietary vitamin C on growth and immune response of Japanese seabass,.,242: 489-500, DOI: 10.1016/ j.aquaculture.2004.08.016.
Alliot, E., Febvre, A., Metailler, R., and Pastoureaud, A., 1974. Besoins nutritifs du bar (L.) Etude du taux de protéine et du taux de lipide dans le régime. Actes Colloq, CNEXO, 1: 215-228.
Association of Official Analytical Chemists (AOAC), 1995.. 16th edition. Association of Official Analytical Chemists, Arlington, VA.
Balage, M., Dupont, J., Mothe-Satney, I., Tesseraud, S., Mosoni, L., and Dardevet, D., 2011. Leucine supplementation in rats induced a delay in muscle IR/PI3K signaling pathway associated with overall impaired glucose tolerance.,22: 219-226.
Chance, R. E., Mertz, E. T., and Halver, J. E., 1964. Nutrition of salmonid fishes: XII Isoleucine, leucine, valine and phenylalanine requirements of chinook salmon and interrelation between isoleucine and leucine for growth., 83: 177-185.
Choo, P. S., Smith, T. K., Cho, C. Y., and Ferguson, H. W., 1991. Dietary excesses of leucine influence growth and body composition of rainbow trout., 121: 1932-1939.
Crozier, S. J., Kimball, S. R., Emmert, S. W., Anthony, J. C., and Jefferson, L. S., 2005. Oral leucine administration stimulates protein synthesis in rat skeletal muscle., 135: 376-382.
de la Higuera,M., 2001. Effcts of nutritional factors and feed characteristics on feed intake. In:. Houlihan, D.,., eds., Blackwell, Oxford, 250-268.
Dardevet, D., Sornet, C., Bayle, G., Prugnaud, J., Pouyet, C., and Grizard, J., 2002. Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-sup- plemented meal., 132: 95-100.
D’Mello, J. P. F., 2003. Adverse effects of amino acids. In:.2nd edition. D’Mello, J. P. F., ed., CABI Publishing, Wallingford, UK, 125-142.
Donato Jr., J., Pedrosa, R. G., de Araujo Jr., J. A., Pires, I. S., and Tirapegui, J., 2007. Effects of leucine and phenylalanine supplementation during intermittent periods of food restriction and refeeding in adult rats., 81: 31-39.
Eller, L. K., Saha, D. C., Shearer, J., and Reimer, R. A., 2013. Dietary leucine improve whole-body insulin sensitivity independent of body fat in diet-induced obese Sprague-Dawley rats., 24 (7): 1285-1294, DOI: org/10.1016/j.jnutbio.2012.10.004.
Forster, I., and Ogata, H. Y., 1998. Lysine requirement of juvenile Japanese flounderand juvenile red sea bream.,161: 131-142.
Freudenberg, A., Petzke, K. J., and Klaus, P. S., 2012. Comparison of high-protein and leucine supplementation in the prevention of metabolic syndrome and related disorders in mice., 23: 1524-1530, DOI: 10.1016/j.jnutbio.2011.10.005.
Garlick, P. J., 2005. The role of leucine in the regulation of protein metabolism., 135 (6 Suppl): 1553S- 1556S.
Hughes, S. G., Rumsey, G. L., and Nesheim, M. C., 1984. Effects of dietary excess of branched-chain amino acids on the metabolism and tissue composition of lake trout ()., 78A: 413-418.
Kaushik, S. J., 1998. Whole body amino acid composition of European seabass (), gilthead seabream () and turbot () with an estimation of their IAA requirement profiles.,11 (5): 355-358, DOI: 10.1016/S0990-7440(98)80007-7.
Khan, M. A., and Abidi, S. F., 2007. Total aromatic amino acid requirement of Indian major carp(Hamilton) fry., 267: 111-118, DOI: 10.1016/j.aquaculture 2007. 02.025.
Kimball, S. R., and Jefferson, L. S., 2006. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis.,136 (1 Suppl): 227S-231S.
Li, A. J., 1996.. China Agriculture Press, Beijing, 21pp.
Luo, Z., Liu, Y. J., Mai, K. S., Tian, L. X., Yang, H. J., and Tan, X. Y., 2005. Dietary l-methionine requirement of juvenile grouperat a constant dietary cystine level., 249: 409-418, DOI: 10.1016/j.aquaculture. 2005.04.030.
López, N., Sánchez, J., Picó, C., Palou, A., and Serra, F., 2010. DietaryL-leucine supplementation of lactation rats results in a tendency to increase lean/fat ratio associated to lower orexigenic neuropeptide expression in hypothalamus.,31: 1361-1367, DOI: 10.1016/j.peptides.2010.03.028.
Macotela, Y., Emanuelli, B., Bang, A. M., Espinoza, D. O., Boucher, J., Beebe, K., Gall, W. C., and Kahn, R., 2011. Dietary leucine–An environmental modifier of insulin resistance acting on multiple levels of metabolism., 6: e21187, DOI: 10.1371/journal.pone.0021187.
Mai, K. S., Wan, J. L., Ai, Q. H., Xu, W., Liufu, Z. G., Zhang, L., Zhang, C. X., and Li, H. T., 2006. Dietary methionine requirement of large yellow croaker,R.,253: 564-572, DOI: 10.1016/j.aquaculture.2005. 08.010.
Millamena, O. M., Bautista, M. N., Reyes, O. S., and Kanazawa, A., 1997. Threonine requirement of juvenile marine shrimp., 151: 9-14, DOI: 10.1016/ S0044-8486(96)01486-x.
Millamena, O. M., Teruel, M. B., Kananzawa, A., and Teshima, S., 1999. Quantitative dietary requirements of postlarval tiger shrimp,, for histidine, isoleucine, phenyla- lanine and tryptophan., 179: 169-179, DOI: 10 1016/S0044-8486(99)00160-x.
Ng, W. K., and Hung, S. S. O., 1995. Estimating the ideal dietary indispensable amino acid pattern for growth of white sturgeon., 1: 85-94, DOI: 10.1111/j. 1365-2095.1995.tb00023.x.
Rieu, I., Balage, M., Sornet, C., Giraudet, C., Pujos, E., Grizard, J., Mosoni, L., and Dardevet, D., 2006. Leucine supplementation improve smuscle protein synthesis in elderly men independently of hyperaminoacidaemia.,575 (1): 305-315, DOI: 10.1113/jphysio.2006.110742.
Rollin, X., 1999.Critical study of indispensable amino acids requirements of Atlantic salmon (L.) fry. PhDthesis. Universitée catholique de Louvain, Louvain, Belgium, 17-30.
Tibaldi, E., and Tulli, F.,1999. Dietary threonine requirement of juvenile european sea bass ()., 175: 155-166, DOI: 10.1016/S0044-8486(99)00029-0.
Toneto, A. T., Salomao, E. M., Sebinelli, M. C. C., and Gomes- Marcondes, 2012. Effects of leucine-rich diet and exercise on inflammatory response produced by the tumour growth in rats., 59: P053, http://dx.doi.org/10.1016/j.cyto. 2012.06.137.
Vianna, D., Resende, G. F. T., Torres-Leal, F. L., Pantaleāo, L. C., Donato, J., and Tirapegui, J., 2012. Long-term leucine supplementation reduces fat mass gain without changing body protein status of aging rats., 28: 182-189, DOI: 10.1016/j.nut.2011.04.004.
Wilson, R. P., and Halver, J. E., 1986. Protein and amino acid requirements of fishes., 6: 225- 244.
Wilson, R. P., William, E. P., and Robinson, E. H., 1980. Leucine, isoleucine, valine and hisidine requrirementf of fingerling channel catfish., 110 (4): 627-633.
Yamamoto,T., Shima, T., and Furutia, H., 2004. Antagonistic effects of branched chain amino acids induced by excess protein bound leucine in diets for rainbow trout ()., 232: 539-550, DOI: 10.1016/S0044- 8486(03)00543-X.
Zeitoun, I. H., Ullrey, D. E., and Magee, W. T., 1976. Quanti- fying nutrient requirements of fish.,33: 167-172.
Zhang, C. X., Mai, K. S., Ai, Q. H., Zhang, W. B., Duan, Q. Y., Tan, B. P., Ma, H. M., Xu, W., Liufu, Z. G., and Wang, X. J., 2006. Dietary phosphorus requirement of juvenile Japanese seabass,., 255: 201-209, DOI: 10.1016/j.aquaculture.2005.11.040.
Zhang, Y., Guo, K., LeBlanc, R. E., Loh, D., Schwartz, G. J., and Yu, Y. H., 2007. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in micemultimechanisma., 56 (6): 1647-1654.
(Edited by Qiu Yantao)
DOI 10.1007/s11802-015-2387-5
ISSN 1672-5182, 2015 14 (1): 121-126
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
(May 6, 2013; revised May 29, 2013; accepted November 19, 2014)
* Corresponding author. Tel: 0086-532-82031943 E-mail: qhai@ouc.edu.cn
杂志排行
Journal of Ocean University of China的其它文章
- The Influence of El Niño on MJO over the Equatorial Pacific
- Research on the Interannual Variability of the Great Whirl and the Related Mechanisms
- Brightness Temperature Model of Sea Foam Layer at L-band
- Parametric Instability Analysis of Deepwater Top-Tensioned Risers Considering Variable Tension Along the Length
- DPOI: Distributed Software System Development Platform for Ocean Information Service
- Nonlinear Contact Between Inner Walls of Deep Sea Pipelines in Buckling Process
