穿山龙多糖DMA 的结构和抗氧化活性研究
2015-01-11李志明罗巅辉王昭晶
李志明,罗巅辉,王昭晶
华侨大学生物工程与技术系,厦门 361021
Oxidation is essential to many organisms for the production of energy to fuel biological processes.However,the uncontrolled production of oxygen derived free radicals is involved in onset of many diseases such as cancer,rheumatoid arthritis and atherosclerosis as well as in degenerative processes associated with aging[1].Many synthetic antioxidants are used as medical materials at the present time.However,most commonly have been suspected of being responsible for liver damage and carcinogenesis[2].Thus,it is essential to develop and utilize effective and natural antioxidants[3].Published data indicated that plant polysaccharides in general had strong antioxidant activities and can be explored as novel and potential antioxidants[4].
Dioscorea nipponica Makino is well known as a traditional edible plant in oriental countries.It has been widely used as Chinese herbal medicine and health food for hundreds of years.We reported on the extraction and purification of a major polysaccharide of D.nipponica using a DEAE Sepharose CL-6B column chromatography and a Sephadex G-100 column chromatography[5].Structural identification of polysaccharides is necessary to better study the relationship between structures and functional properties of polysaccharides and effectively exploit these potential substances.However,there are only a few studies on its chemical composition and structures,and its detailed structure is not precisely known.
In this study,we reported on the extraction and purification of a major polysaccharide DMA of D.nipponica Makino using a DEAE Sepharose CL-6B column chromatography.In addition,the structural characteristics and antioxidant activities of the major polysaccharide DMA were also identified.
Material and Methods
Materials and chemicals
Dried underground part of D.nipponica was purchased from a local drugstore(Quanzhou,Fujian Province,China).The material was identified by Professor J.F.Liu,Huaqiao University,Fujian,China.Monosaccharide standard samples(G-8270,Sigma Co.,St Louis,MO,USA),Nitro Blue Tetrazolium(NBT,3069B117,Amresco Inc.,Solon,OH,USA),Nicotinamide Adenine Dinucleotide Hydrogen(NADH,6109B08,Amresco Inc.,Solon,OH,USA)and Phenazine Methosulfate(PMS,101413172,Fluka Inc.,St Louis,MO,USA)were purchased from a local agent(Taijing Co.,Xiamen),while DEAE Sepharose CL-6B was from the Pharmacia Co.(Sweden).All other reagents used were of analytical grade.
Instruments
High performance liquid chromatography(HPLC)was performed on a HP1100 instrument(Agilent Component,USA).Fourier transform infrared spectrophotometer(FT-IR)was Nicolet Nexus 470(NICOLET Component,USA).GC-FID and GC-MS analyses were performed on a HP5890 GC instrument and a HP5890-5972 GC-MS instrument,respectively(Agilent Component,USA).NMR spectrometer was from Bruker Inc.(DRX500,Switzer-land).
Methods
Preparation of polysaccharide DMA
D.nipponica sample was extracted with distilled water at 90 ℃for 2.5 h.The soluble polymers were separated from residues by filtration,and extracts were concentrated.The supernatant was collected and precipitated by the addition of four times volume ethanol.After overnight precipitation at 4 ℃,the sample was centrifuged at 3,500 rpm for 15min.The sample was washed successively with ethanol,and dried at reduced pressure,giving a crude polysaccharide(DM).The yield was calculated using the equation:DM weight/sample weight×100%.DM(1 g)was dissolved in 10 mL of distilled water,centrifuged and then the supernatant was injected to a column(4.6 cm×30 cm)of DEAESepharose CL-6B equilibrated with distilled water for 500 mL at 4 mL/6 min.A major polysaccharide fraction was collected,named DMA.
Total carbohydrate and protein of these polysaccharides were determined by the phenol-sulfuric acid and Bradford,respectively[6].GC-FID was used for identification and quantification.First,the polysaccharide(10 mg)was dissolved in 1 mL of a 1 M HCl-Methanol,removed air with nitrogen,hydrolyzed at 80 ℃for 20 h,and then neutralized by KOH-Methanol.Derivation was then carried out using the trimethylsilylation reagent.The monosaccharide compositions of DMA were determined by gas chromatography equipped with a column HP-5(30 m×0.25 mm×0.25 μm)and the flame-ionization detector.Temperatures-programmed GC-FID measurements were carried out from 180 ℃to 220 ℃(held for 2 min)at a rate of 8 ℃/min,and then to 250 ℃(held for 2 min).The molecular weight of the polysaccharide DMA was determined by a gel-filtration chromatographic column of Shodex Sugar KS-804(SHOWA DENKO K.K,Japan)with a high-performance liquid chromatography instrument(Agilent 1100,USA)maintained at a temperature of 50 ℃,eluted with the distilled water at a flow rate of 1.0 mL/min and detected by a refractive index detector.Preliminary calibration of the column was conducted using several standard samples of different molecular weight(T2000,T10,T40,T70,T500 and Glucose)[6].
Structural analysis
IR spectrum of the polysaccharide was determined using a Fourier transform infrared spectrophotometer(Nicolet Nexus 470,NICOLET Component,USA).The purified polysaccharide was ground with KBr powder and then pressed into pellets for FTIR measurement in the frequency range of 4000-400 cm-1[7].
For analytical purpose of Periodate oxidation-Smith degradation,25 mg of DMA was dissolved,and four samples were obtained and dried out for the GC-FID analysis[5].
For methylation analysis and GC-MS analysis,polysaccharide(40 mg)was dissolved in dimethylsulfoxide(DMSO,12 mL),removed air with nitrogen,stirred for 24 h at 25 ℃,and then sodium hydroxide-DMSO combined solution(12 mL)was added.After blending well 30 min and reacting with 7.2 mL of methanol iodide for 7 min exactly,the product was dialyzed against running water and distilled water for 24 h,respectively.The solution was concentrated to 10 mL,treated thrice with 10 mL chloroform,and fully shocked to extract the polysaccharide,after watered,dried over Na2SO4for 24 h,filtered and evaporated to 1 mL,then examined by IR spectrum.The desiccated sample was subjected to 1 mL of 88% formic acid,suffused with nitrogen,kept at 100℃for 4 h,got rid of excess formic acid by methanol,and dried in vacuum desiccators.The above sample was hydrolyzed in 2 M trifluoroacetate(TFA)for 6 h at 100 ℃,treated with ethanol then distilled water,reduced with NaBH4for 24 h,and then acetylated with acetic anhydride-pyridine(1∶1)at 100 ℃for 2 h.The resulting acetates were subjected to GC-MS analyses.GC-MS was performed on a HP5890 instrument(Agilent Component,USA)with a Rtx-5ms column(30 m×0.25 mm×0.25 μm),and at temperatures programmed from 160 to 250 ℃ at 8 ℃/min.Linkages were identified on the basis of relative retention time and fragmentation pattern.Peaks of methylated products were identified by mass spectra.The molar ratios for each sugar were calibrated using the peak areas and response factor[8].
For NMR measurements,30 mg DMA was dried in a vacuum over P2O5for several days,exchanged with deuterium by lyophilizing with D2O for several times,and then dissolved in D2O(99.9%).The1H and13C NMR spectra experiments were recorded at 500 MHz and 125 MHz on a spectrometer(BRUKER DRX500,BRUKER Component,Swiss),respectively.The 2D COSY and HSQC NMR were performed using standard software.The1H NMR chemical shifts were referenced to residual HOD at δ 4.71 ppm.The13C spectrum was recorded at δ 31.05 ppm.HSQC data was recorded after a 50 ms delay to allow for the evolution of longrange couplings.
Antioxidant activities assay
The superoxide radical assay and the hydroxyl radical ability were measured by the methods of Ghiselli and Robak with a minor modification,respectively[5].
The scavenging ability for DPPH of DMA was investigated.Briefly,samples or Vc were dissolved in distilled water at 0,2.5,5,10,20,40,80 and 100 μg/mL.The sample solution(0.1 mL)or distilled water(0.1 mL,control)was mixed with 2 mL of 0.3 mM DPPH,and incubated for 20 min at 25 ℃.The absorbance of the mixture was measured at 517 nm.The scavenging ability for DPPH of DMA was calculated using the following equation:(1-absorbance of sample/absorbance of control)×100%.
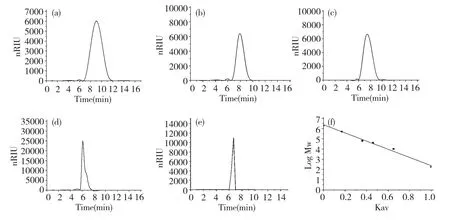
Fig.1 HPLC chromatogram of T10(a),T40(b),T70(c),T500(d),DMA(e)and standard curve of molecular weight(f)
Results and Discussion
Isolation,purification and composition analysis of DMA
The crude polysaccharide was isolated from the hot-water extract of D.nipponica with a yield of 8.05%.After fractioned on DEAE-Sepharose CL-6B column,DMA(30.6%)was obtained.DMA was one peak and a kind of polysaccharide by HPLC with Sugar KS-804 column(Fig.1),which was also confirmed by NMR.The homogeneity of DMA was not good for the asymmetric peak.The average molecular weight of DMA was 22.9×104according to standard curve(y=-3.67x+6.356).The polysaccharide content of DMA was 98.2% by the phenol-sulfuric acid,and DMA was mainly consisted of glucose by GC-FID analysis of DMA hydrolyzed by TFA and standard samples(Fig.2 and Table 1).
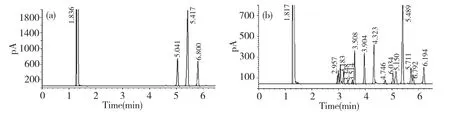
Fig.2 GC-FID chromatograms of DMA hydrolyzed by TFA(a)and standard samples(b)
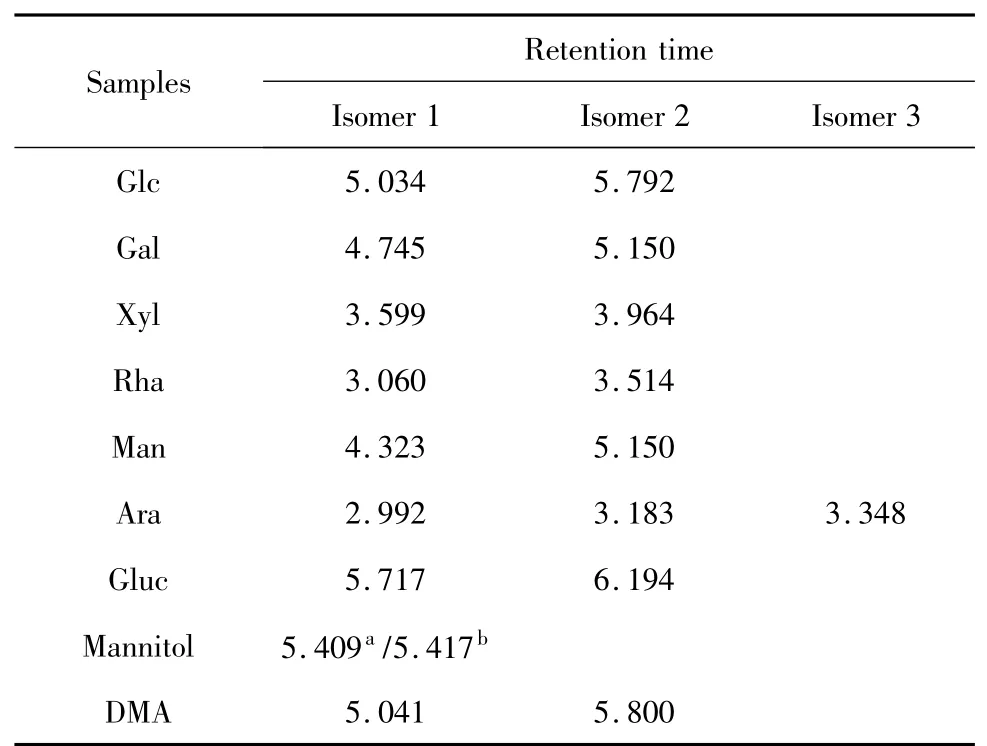
Table 1 Retention time of standard samples and DMA
Structural characterization of DMA
The polysaccharide of DMA showed abundance HIO4uptake,while it was oxidized.The consumption of HIO4(0.293 m mol)was two times than the amount of formic acid(0.143 mmol)that was produced after 36 h of the treatment,indicating the existing of large amounts of monosaccharide which were 1→linked or 1→6 linked of DMA.In addition,it should be concluded that sugar residues oxidized and linkages of(1→)-glycosidic linkages accounted for 97.42%.The oxidized products were hydrolyzed and examined by GC-FID.Glucose was absent,then it may be inferred that glucose was all linkages that can be oxidized,namely(1→)-glycosidic linkages or(1→6)-glycosidic linkages.Results of smith-degradation analysis of fractions were obtained.There was no precipitation in the sack and no substances in the supernatant of sack,indicating that the backbone of DMA should be all oxidized by HIO4.In the same time,the linkages of backbone could be arrayed by(1→6)-glycosidic linkages,(1→2)-glycosidic linkages or(1→2,6)-glycosidic linkages,according to the presence of glycerin out of sack.
The IR spectrum of DMA displayed a broad stretching intense characteristic peak at around 3407 cm-1for the hydroxyl group(Fig.3a).The IR spectrum of methylated DMA(Fig.3b)showed that no absorption peak of hydroxyl group identified the complete methylation.The analysis of fraction DMA showed the presence of 2,3,4-Me3-Glc(Table 2).The methylation analysis of DMA indicated that 2,3,4-Me3-Glc was the major component of the main-chain structure.On the basis of the results obtained above,it was possible to conclude that a repeating unit of DMA contained a main-chain composed of(1→6)-linked-glucose.

Fig.3 IR spectra of DMA(a)and methylated DMA(b)
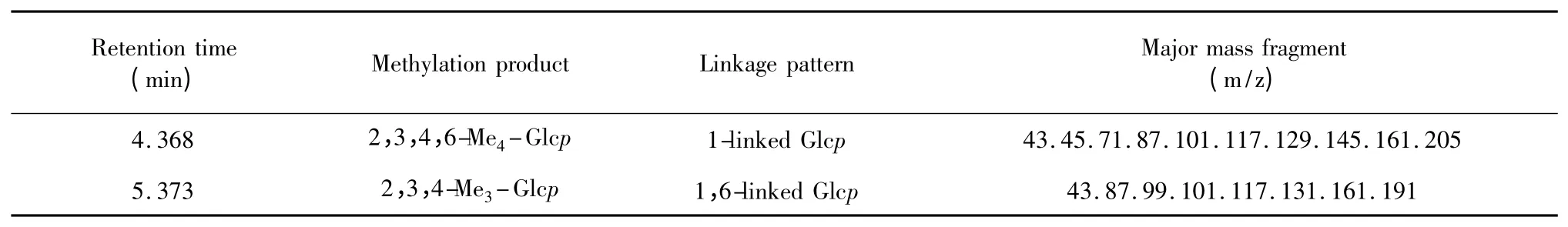
Table 2 Methylation analysis of DMA
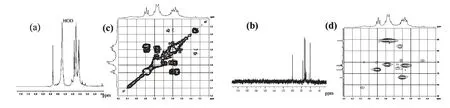
Fig.4 1H NMR(a),13C NMR(b),1H-1H COSY(c)and Part of HSQC(d)spectra of DMA
The1H(Fig.4a)and13C(Fig.4b)NMR experiments were carried out.The 500 MHz1H NMR spectrum showed only one signal at δ 5.30 ppm.Since it did not provide enough information about the anomeric configuration,a one-bond C-1-H-1 heteronuclear NMR experiment was carried out.All the1H and13C signals were assigned using 2D-COSY(Fig.4c)and HSQC(Fig.4d)NMR experiments.The residue was designated as A according to its increasing anomeric shift.The proton chemical shifts of A were assigned from H-1 to H-6 by 2D-COSY spectra[9,10].The carbon chemical shifts from the C-1 to C-6 for A were assigned from the HSQC spectrum,and these correspond nearly to the standard values of methyl glycosides of D-glucose.The anomeric signals for moiety A at δ 5.30>5.0 ppm and the C-1 signal of A at 99.64 ppm confirmed by the HSQC experiment indicated that A residue was α-Dpyranose[11].The downfield shifts of C-6 carbon signal with respect to standard values indicated that the moiety A was linked at these positions[11].Thus,considering the results of methylation analysis and NMR experiments,it may be concluded that A was pyranoid→6)-α-D-Glcp-(1→.Based on all these evidences,the repeating unit in polysaccharide DMA was assigned as→6)-α-D-Glcp-(1→.
Antioxidant activities
DMA had the scavenging effect for hydroxyl radicals.As shown in Fig.5,the scavenging effects increased with increasing concentration.Scavenging effects of DMA were 0% to 28.9% at amount of 0.25 to 4 mg among the reaction system,respectively,and that of Vitamin C was about 0 to 43.09%.This result proved that polysaccharide DMA from D.nipponica had significant effect on scavenging hydroxyl radical,and that was lower than Vc.DMA had no abilities to scavenge superoxide radical and DPPH.
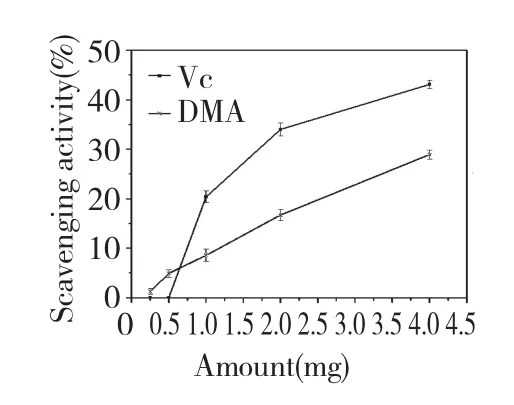
Fig.5 Scavenging effects of DMA and Vc on hydroxyl radical
Conclusion
According to the results above,it was concluded that the crude water extracting polysaccharide of D.nipponica contained the water extractable polysaccharide(DMA)purified by DEAE Sepharose CL-6B.The IR,periodate oxidation-smith degradation,methylation and NMR analyses results indicated that DMA mainly composed of→6)-α-D-Glcp-(1→.In addition,DMA had a scavenging activity of hydroxyl radical.
1 Mau JL,Lin HC,Song SF.Antioxidant properties of several specialty mushroom.Food Res Int,2002,35:519-526.
2 Qi HM,Zhang QB,Zhao TT,et al.Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa(Chlorophyta)in vitro.Int J Biol Macromol,2005,37:195-199.
3 Nandita S,Rajini PS.Free radical scavenging activity of an aqueous extract of potato peel.Food Chem,2004,85:611-616.
4 Jiang YH,Jiang XL,Wang P,et al.In vitro antioxidant activities of water-soluble polysaccharides extracted from Isaria farinosa B05.J Food Biochem,2005,29:323-335.
5 Luo DH.Identification of structure and antioxidant activity of a fraction of polysaccharide purified from Dioscorea nipponica Makino.Carbohyd Polym,2008,71:544-549.
6 Luo DH,Fang BS.Structural identification of ginseng polysaccharides and testing of their antioxidant activities.Carbohydr Polym,2008,72:376-381.
7 Kumar CG,Joo HS,Choi JW,et al.Purification and characterization of an extracellular polysaccharide from haloalkalophilic Bacillus sp.I-450.Enzyme Microb Tech,2004,34:673-681.
8 Wang GY,Liang ZY,Zhang LP,et al.Studies on the structure of JS(1)-the water soluble polysaccharide isolated by alkaline from Hippophae rhamnoides L.Chem J Chin Univ,2001,22:1688-1690.
9 Pan D,Wang LQ,Chen CH,et al.Structure characterization of a novel neutral polysaccharide isolated from Ganoderma lucidum fruiting bodies.Food Chem,2012,135:1097-1103.
10 Yang Y,Zhang JS,Liu YF,et al.Structural elucidation of a 3-O-methyl-D-galactose-containing neutral polysaccharide from the fruiting bodies of Phellinus igniarius.Carbohydr Res,2007,342:1063-1070.
11 Mondal S,Chakraborty I,Rout D,et al.Isolation and structural elucidation of a water-soluble polysaccharide(PS-I)of awild edible mushroom,Termitomyces striatus.Carbohydr Res,2006,341:878-886.
