微生物酶催化碳氢键的不对称氧化反应合成手性4-氯二苯基甲醇*
2014-09-10陈永正卓俊睿郑代军付少彬
陈永正,杨 敏,卓俊睿,郑代军,付少彬
(遵义医学院药学院,贵州遵义 563099)
1 Introduction
Chiral sec-alcohols were key intermediate in the synthesis of fine chemicals,pharmaceuticals,and agrochemicals[1].Chiral benzhydrol was one kind of important building block for synthesis of orphenadrine 1,neobenodine 2,carbinoxamine 3[2].Moreover,chiral benzhydrol was also used as an intermediate of some potent anti-inflammatory drugs 4 and ODE-Ⅳ inhibitor.
Currently,numerous syntheses of chiral benzhydrol mainly depended on reduction of ketones,hydrolysis of esters and kenetic resolution of racemic alcohols with chemical catalyst or biocatalyst[3].For example,a new γ - amino thiol was designed for synthesis of chiral benzhydrol by asymmetric arylation of aromatic aldehydes[4].The addition reaction of organoboronic acids with aldehydes catalyzed by CoI2/(R,R)-BDPP also give chiral benzhydrol in excellent yields with 90% ~ 99%ee[5-6].Truppo group reported that a reductase catalyzed the reduction of di- aryl ketones to produce chiral benzhydrol[7].
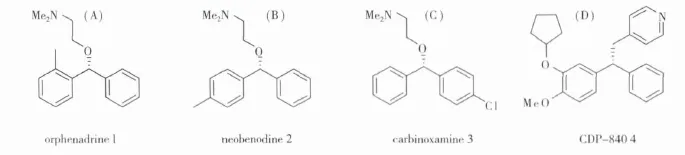
Fig 1 Synthetic application of chiral benzyldrols 1-4
Enantioselctive hydroxylation of C-H bonds gave a direct access for synthesis of chiral sec-alcohols.Though regio- and stereoselective hydroxylations provided an easy way to synthesize chiral alcohols,but chemical hydroxylations often suffer from poor chemo - ,regio - ,and enantioselectivity[8].Recent years,some purified enzymes and microbial whole-cell catalysts have been developed for hydroxylation of C - H bonds[9-12].However,there are no reports by using whole-cell biocatalyst to catalyze the enantioselective hydroxylation of CPM to chiral CPML.Herein,we reported asymmetric hydroxylation of CPM to CPML,which are key intermediate of levocetirizine.The whole cells of P.monteilii TA -5 could catalyze asymmetric hydroxylation at C-H bond of benzylic position with high enantioselectivity(Scheme 2).It provides a whole cells-based oxidation way for preparation of chiral CPML with cleaner and greener access.

Fig 2 Biocatalytic synthesis of chiral(S)-CPML
2 Experiments
2.1 Chemicals and microbial strains All chemical reagents and solvents were purchased from J&K and used without further purification.However,racemic 4-chlorobenzhydrol(98%)was purchased from TCI,and other chemicals were purchased from J&K or Merck without further purification.
2.2 Analytical methods The ee values of products were determined using a ShimadzuTM Prominence HPLC on a DaicelTMAD-H chiral column(250×4.6 mm,5 μm)at 25℃ with a flow rate of 0.5 mL/min and UV detection at 220 nm.The configuration was assigned with racemic 4-chlorobenzhydrol and also established by comparison with the references[6].2.3 Biohydroxylation sprocedure of 4 - chlorodipheylmethane 1 μL inoculated P.monteilii TA - 5 was transferred from M9 agar plate into LB liquid medium.The culture was allowed to grow for 24 h at 30℃and 300 rpm.1 mL LB seed culture was then transferred into 50 mL M9 medium supplemented with trace element in 250 mL conical flask containing 1%(v/v)toluene in a plastic tube.The cells were grown at 30℃and 250 rpm for 21h and harvested by centrifugation at 8000 rpm for 10 min.The cells of microorganism were suspended in 5 mL 50 mM KH2PO4-K2HPO4buffer(pH 7.0).Substrate was added and the mixtures were shaken at 30℃and 300 rpm for 24 h.1 mL samples were taken and extracted with 1 mL ethyl acetate,and analyzed by chiral HPLC.
2.4 Optimization of reaction conditions The cells of P.monteiliiTA -5 was suspended in 5 mL 50 mM KH2PO4-K2HPO4buffer(pH 7.0)to a density of 2~10 g dcw/L.2~8 mM CPM was added and the mixtures were shaken at 30℃ ~35℃ and 300 rpm for 24 h,respectively.1 mL samples were taken and extracted with 1 mL ethyl acetate.The organic phase was analyzed by chiral HPLC.
2.5 Spectral and ee data for the biosynthesized compounds Compound CPML:DaicelTMAD-H chiral column(250 ×4.6 mm,5 μm)at 25℃ with a flow rate of 1 mL/min and UV detection at 220 nm.Retention time:16.6 min for(R)- CPML and 18.2 min for(S)-CPML(AD-H column,10%isopro-panol:90%n-hexane).

Fig 3 Cell growth and hydroxylation activity of P.monteilii TA-5
3 Results and discussions
3.1 Cell growth and hydroxylation activity of P.monteilii TA-5 The cells of P.monteilii TA-5 were grown in a M9 medium with the vapor of toluene.Samples were taken at different time points for the determination of optical density at 450 nm.At the same time,cells in the samples were harvested and suspended in buffer for the hydroxylation of 5 for 1h to examine the specific activity.As shown in Scheme 3,cells grew fast and reached 5.0 g dcw/L after 19 h with a hydroxylation activity of 0.08 U/g dcw.The cell growth reached a late exponential phase at 23 h,and after that time point the specific activity of the cells slightly decreased.
3.2 Optimization of reaction conditions on the hydroxylation of CPM Following the interesting results above,the reaction parameters were optimized,such as substrate concentration,cell density,reaction temperature,and pH value(Table 1 and Table 2).As shown in Table 1,bioxidation of 2~16 mM CPM at a cell density of 10 ~60 g dcw/L and pH 7.0 for 24 h(entries 1~5).The best results were obtained with 2 mM of CPM,leading to the formation of 3.2%yield of(S)- 1a in 78%ee.Moreover,when substrate concentration was increased from 2 mM to 16 mM,the conversion and enantioselectivity of(S)-CPML were decreased.In addition,different cell density had investigated for the effect of this biotransformation,no obvious positive effect on yield(entries 1~6).Moreover,pH of 7.0 was shown to be better than pH of 6.0 or 8.0.Moreover,reaction at 30℃ resulted in better conversion and enantioselectivity at 35℃ or 25℃.For example,(S)-1a in 90%ee was obtained when reaction temperature was increased from 30℃to 35℃.
To further know better view of this whole cell catalyst for hydroxylation of CPM with cosolvent system,10 kinds of cosolvents 10%(v/v)were investigated.Comparing the control experiments,a little better yield of S-CPML was obtained by using 10%(v/v)glycerol and glycol as coslovents(entry 24 and 26).10%glycol cosolvent system was chosen as the best reaction medium.
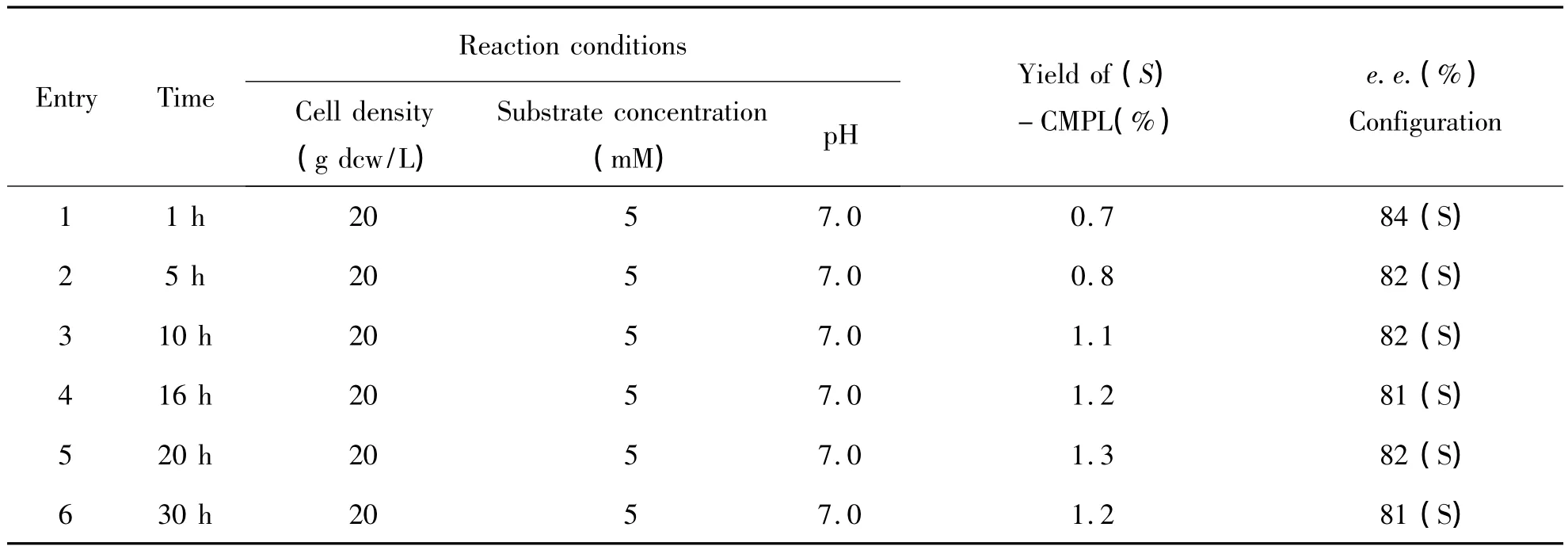
Table 1 The effect of reaction time on the catalytic reaction with P.monteiliiTA-5

Table 2 The effect of reaction condition on the catalytic reaction with P.MonteiliiTA-5
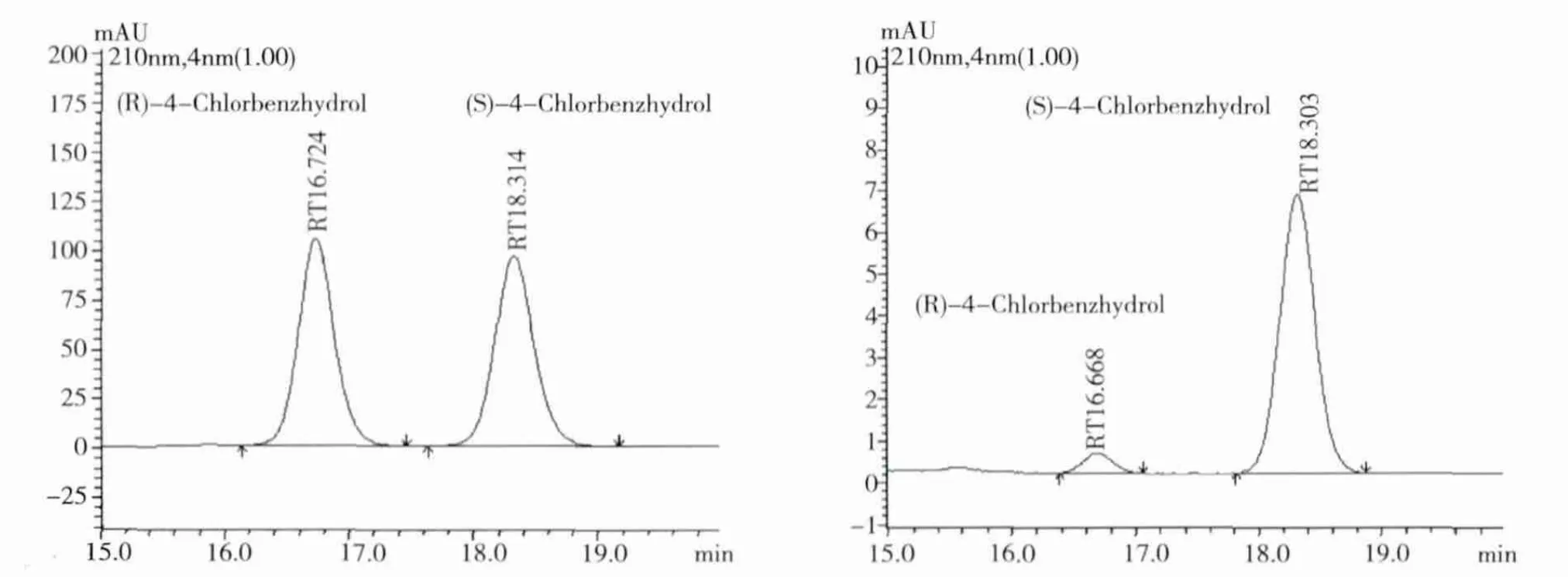
Fig 4 Chiral HPLC chromatogram of rac-CPML(A)and(S)-CPML(B)
3.3 Optimization of reaction conditions on the hydroxylation of CPM On the basis of these results,efficient productions of optically active S-CPML at 50 mL(300 mM Na2HPO4-NaH2PO4buffer,pH 9.0)scale using whole cells.P.monteilii TA -5 was investigated,S -CPML was obtained with 5.5%isolated yield in 89%e.e.in the presence of 10%(w/v)glycol after 24h incubation.Moreover,the configuration of S-CPML was assigned by racemic samples and references[6].
4 Conclusion
A biocatalytic protocol is described for enantioselective oxidation of CPM with 89%ee by P.monteilii TA-5.Under optimized conditions,the performance of the strain as a whole-cell catalyst is unique.P.monteilii TA-5 show the ability for directly oxidation to prepare chiral 4 -chlorobenzhydrol.It showed positive developments in the direct synthesis of chiral 4-chlorobenzhydrol using microbial cells.For more detailed information on enzyme is essential and in progress.
[1]Yang X F,Hirose T,Zhang G Y.Catalytic enantioselectivearylation of aryl aldehydes by chiral aminophenol ligands[J].Tetrahedron:Asymmetry,2009,20(4):415 -419.
[2]Alexander R P,Warrellow G J,Eatonv MAW,et al.CDP840.A Prototype of a Novel Class of Orally Active Anti- Inflammatory Phosphodiesterase 4 Inhibitors.Bioorg[J].Med Chem Lett,2002,12(11):1451 -1456.
[3]Lee C T,Lipshutz B H.Nonracemic Diarylmethanols From CuH - Catalyzed Hydrosilylation of Diaryl Ketones[J].Org Lett,2008,10(19):4187 -4190.
[4]Lu G,Kwong F Y,Ruan J W,et al.Highly Enantioselective Addition of In Situ Prepared Arylzinc to Aldehydes Catalyzed by a Series of AtropisomericBinaphthyl-Derived Amino Alcohols[J].Chem Eur J,2006,12(15):4115-4120.
[5]Liu X Y,Wu X Y,Chai Z,et al.Highly Effective and Recyclable Dendritic Ligands for the Enantioselective Aryl Transfer Reactions to Aldehydes[J].J Org Chem,2005,70(18):7432-7435.
[6]Karthikeyan J,Jeganmohan M,Cheng C H,et al.Cobalt-Catalyzed Addition Reaction of Organoboronic Acids with Aldehydes:Highly Enantioselective Synthesis of Diarylmethanols[J].Chem Eur J,2010,16(30):8989 -8992.
[7]Homann MJ,Vail R B,Previte E,et al.Rapid identification of enantioselective ketone reductions using targeted microbial libraries[J].Tetrahedron,2004,60(3):789 -797.
[8]Andreas L,Karsten S,Christian W,et al.Industrial Biotransformations(2nd ed.).John Wiley & Sons,2006,p.556.
[9]Yadav S,Yadav RSS,Yadava S,et al.Stereoselective hydroxylation of ethylbenzene to(R)-1-phenylethanol using mycelia of Aspergillus niger as catalyst[J].Catal.Commun,2011,12(9):781 -784.
[10]Kim K,Oh D,Production of hydroxy fatty acids by microbial fatty acid - hydroxylation enzymes[J].Biotechnol Adv,2013,31(8):1473 -1485.
[11]Huang T,Rehak L,Jander G.meta-Tyrosine in Festuca rubra ssp.commutata(Chewings fescue)is synthesized by hydroxylation of phenylalanine[J].Phytochemistry,2012,75:60-66.
[12]Chen Y Z,Lie F,Li Z.Enantioselective Benzylic Hydroxylation with Pseudomonas monteiliiTA-5:A Simple Method for the Syntheses of(R)-Benzylic Alcohols Containing Reactive Functional Groups[J].Adv Synth Catal,2009,351(13):2107 -2112.
