荧光化学传感器和化学计量型传感器用于离子识别的研究进展
2014-07-18喻艳华付成
喻艳华,付成
(江汉大学交叉学科研究院,光电化学材料与器件省部共建教育部重点实验室(江汉大学),湖北武汉430056)
荧光化学传感器和化学计量型传感器用于离子识别的研究进展
喻艳华,付成
(江汉大学交叉学科研究院,光电化学材料与器件省部共建教育部重点实验室(江汉大学),湖北武汉430056)
荧光检测相对于放射性核素检测是一种高灵敏度、低成本、操作方便的化学检测技术,可以用于检测多种化学物质。介绍了荧光化学传感器和化学计量型荧光探针的基本原理。阐明了通过“点击”化学(Cu(I)催化下炔基与叠氮形成稳定的1,2,3-3氮唑化合物)合成荧光化学传感器和化学计量型荧光探针的应用,主要从3个方面展开,包括阴离子识别,阳离子识别以及阴、阳离子对的识别。用于阳离子识别的荧光基团重点介绍了氟化硼二吡咯(BODIPY)、苯并噻二唑和香豆素。
离子识别;化学传感器;化学计量型传感器;荧光检测;三氮唑
0 Introduction
A fluorescent chemosensor is an integrated mo⁃lecular device which can convert a molecular recog⁃nition event to a detectable physicochemical signal. It comprises a recognition moiety(ionophore)and a transducer,generally a fluorophore,linked with/with⁃out spacer(Fig.1)[1].The recognition moiety mainly determines the binding selectivity and sensitivity to⁃ wards a given analyte often through noncovalent in⁃teraction,such as hydrogen bonding,metal complex⁃ation,π-π stacking,hydrophobic forces and electro⁃static interactions[2].In some cases,some atoms from the fluorophore may participate to the binding pro⁃cesses,this is called integrated chemosensor.The fluorophore acts as the signal transducer,which con⁃vert the recognition event into photophysical chang⁃es,such as absorption,fluorescent intensity,fluores⁃cence quantum yield and lifetime.These changes are due to the perturbation of photoinduced process⁃es such as electron transfer[3],charge transfer[4],ener⁃gy transfer[5],and formation of excimer[6].
Chemodosimeters represent another approach for the detection of analytes by chemical processes[7-10]. Compared to the principle of chemosensor which is based on coordination events,chemodosimeter in⁃volves chemical reactions induced by a specific ana⁃lyte(such as an anion,a cation or a neutral mole⁃cule).These reactions result in a significant chemi⁃cal transformation including both the breaking and formation of several covalent bonds.This process generally irreversible,reflects a cumulative response related directly to the concentration of the analyte, and shows high selectivity and sensitivity[1].

Fig.1Illustration of a chemosensor
Among the modular approaches offering the pos⁃sibility for rapidly constructing molecular sensor li⁃braries,Cu(I)-catalyzed Huisgen 1,3-dipolar cyclo⁃addition of azides and terminal alkynes(CuAAC) have been increasingly used[11-15].In addition to the synthetic simplicity,the resulting 1,2,3-triazole group can play several important roles in molecular sensing:(1)act as a linker/spacer[16];(2)extend the π-system of an aromatic moiety to create new fluoro⁃phores[17];(3)contribute to the binding of target ana⁃lytes through the nitrogen atoms in the fused triazole ring[18].
In the following review,we chose to classify the chemosensors and chemodosimeters containing tri⁃azole according to the nature of their target analyte.
1 Cation Recognition
Due to the fundamental roles that cations play in biological,chemical and environmental processes, considerable efforts have been directed towards the synthesis of systems able to detect them with high se⁃ lectivity and sensitivity[19].Of these methods,fluores⁃cence-based sensors prepared using"click"chemis⁃try have undoubtedly gained more and more atten⁃tion.The majority of"click"based sensors share overall structure consisting of a binding pocket to which a fluorophore is attached via the triazole link⁃er.Since a variety of fluorophores have been used as sensors,we will discuss the systems based on 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY),benzothiadiazole and coumarin which have excellent fluorescent properties.
1.1 BODIPY-based sensors
BODIPY dyes tend to be strongly UV-absorb⁃ing small molecules that emit relatively sharp fluo⁃rescence peaks with high quantum yields.They are relatively insensitive to the polarity and pH of their environment and are reasonably stable to physiologi⁃cal conditions.Small modifications to their struc⁃tures enable tuning of their fluorescence characteris⁃tics;consequently,these dyes are widely used as sen⁃sors and for proteins labeling[20-22].
Wuandco-workers[23]have developeda BODIPY-based colorimetric and fluorometric che⁃mosensor for Hg(II)ion detection.A monostyryl BODIPY-based fluorescent chemosensor 1 append⁃ed with two triazole units indicates the presence of Hg2+among other metal ions(Ag+,Ca2+,Cd2+,Co2+, Cu2+,Fe2+,K+,Mg2+,Mn2+,Ni2+,Pb2+and Zn2+)in v(acetonitrile):v(water)(9∶1)with high selectivi⁃ty by color change and red emission(650 nm).Upon Hg2+binding,theabsorptionbandof1is blue-shifted by 29 nm(from 606 to 577 nm)due to the inhibition of the intramolecular charge transfer from the aniline nitrogen to the BODIPY,resulting in a color change from blue to purple.In addition, fluorescence enhancement is observed with 1 in the presence of Hg2+.1H-NMR studies indicated that Hg2+binding occurs mainly through the nitrogen at the triazole ring to form the complex 2(Fig.2).
A similar structure of chemosensor based on a distyryl BODIPY with bis(1,2,3-triazole)amino 3 was reported by D K P Ng′s group(Fig.3)[24].This chemosensor has been prepared by Knoevenagel con⁃densation followed by"click"reaction.3 selectivelybinds to Cu2+and Hg2+ions in v(CH3CN)∶v(H2O)(1∶1)to give remarkably blue shifted electronic ab⁃sorption and fluorescence bands as a result of inhibi⁃tion of the intramolecular charge-transfer process upon binding to these metal ions.Change in absorp⁃tion of 3 from green to grey–blue(for Hg2+)or deep blue(for Cu2+),can be easily seen by the naked eye. The emission band was blueshifted by 40 nm(from 726 to 686 nm)upon the addition of Hg2+ions,and by 82 nm(from 726 to 644 nm)in presence of Cu2+. The binding stoichiometry between this probe and Cu2+or Hg2+ions has been determined to be 1∶2 by a Job plot of the fluorescence data,with a binding con⁃stant of(6.2±0.6)×109M-2and(1.1±0.1)×109M-2, respectively.

Fig.2Structure of 1 and complex 2

Fig.3Structure of distyryl BODIPY with bis(1,2, 3-triazolyl)amino derivative 3
1.2 Benzothidiazole-based sensors
Benzothiadiazole derived molecules are widely investigated nowadays due to their well-known pho⁃tophysical properties such as high extinction coeffi⁃cient,intense fluorescence,and large Stokes shift[25-27].
J Xie′s group[12]has synthesized a new multi⁃chromophoric cyclodextrin substituted with benzothi⁃adiazolyl-triazole moiety 6(Scheme 1)by a one-pot "click"reaction between azido-cyclodextrin 4 and TMS-ethynyl benzothiadiazole 5 in the presence of a CuSO4/ascorbate mixture and TBAF for in situ depro⁃tection of TMS group.6 exhibits an absorption band at 372 nm and an emission band at 468 nm.It has high selectivity towards Ni2+by complete quenching of fluorescence emission among a series of cations in CH3CN solution.The association constant for the 1∶1 complex was determined to be 2.88×107M-1
As a continuing program on the development of novelfluoroionophoresandspecialcoordination properties of 1,2,3-triazoles in metal ion sensing, J Xie′s group has attempted to improve the water sol⁃ubility of the BTD-based ligands by incorporation of amino acids through"click"reaction,in order to realize selective metal ions sensing in aqueous solu⁃tion.The benzothiadiazolyl-bis-triazoyl amino acid derivative 7(Fig.4)has been prepared,which exhib⁃its an absorption band at 391 nm and an emission band at 541 nm,it has a significant selectivity to⁃wards Cu2+in"on-off"type response in buffer solu⁃tion at pH 7.4[28].
1.3 Coumarin-based sensors
Coumarins,which contain a benzopyrone core, have many advantages including high fluorescence quantum yield,large Stokes shift and excellent light stability.Therefore coumarins have been used for a wide range of applications,such as fluorescent pH probes[29],detection of hydrogen peroxide[30],and ni⁃troxide[31].
Chung′s group[32]has reported a series of triazo⁃lyl coumarin derivatives 8-11,which were synthe⁃sized as fluorescent sensors to study their binding ability and selectivity toward metal ions(Fig.5). 8,9,10 and 11 exhibit absorption bands at 344, 328,367 and 367 nm and emission bands at 416,395,423 and 427 nm,respectively.Ligand 10, which contains carbonyl linker between the triazole and the coumarin moiety,exhibited a high selectivity toward Hg2+in v(MeOH)∶v(CHCl3)(9∶1)with fluo⁃rescent enhancement.Interestingly,10 was found to bindtwoHg2+cationatahighconcentration(>12.5 mM) of Hg(ClO4)2.In contrast,11,in which position 4 of the triazole unit was substituted by a benzyl group in⁃stead of the 4-tert-butylphenoxymethyl group used in 8-10,showed a binding stoichiometry toward on⁃ly one Hg2+.Fluorescent sensing,IR,and1H-NMR titration results of ligands 8-11 revealed that not on⁃ly the carbonyl C=O but also the ether group of the 4-tert-butylphenoxymethyl of 10 assisted the tri⁃azole nitrogen atoms in the complexation of Hg2+to form a 1∶2 complex 12(Scheme 2)[32].
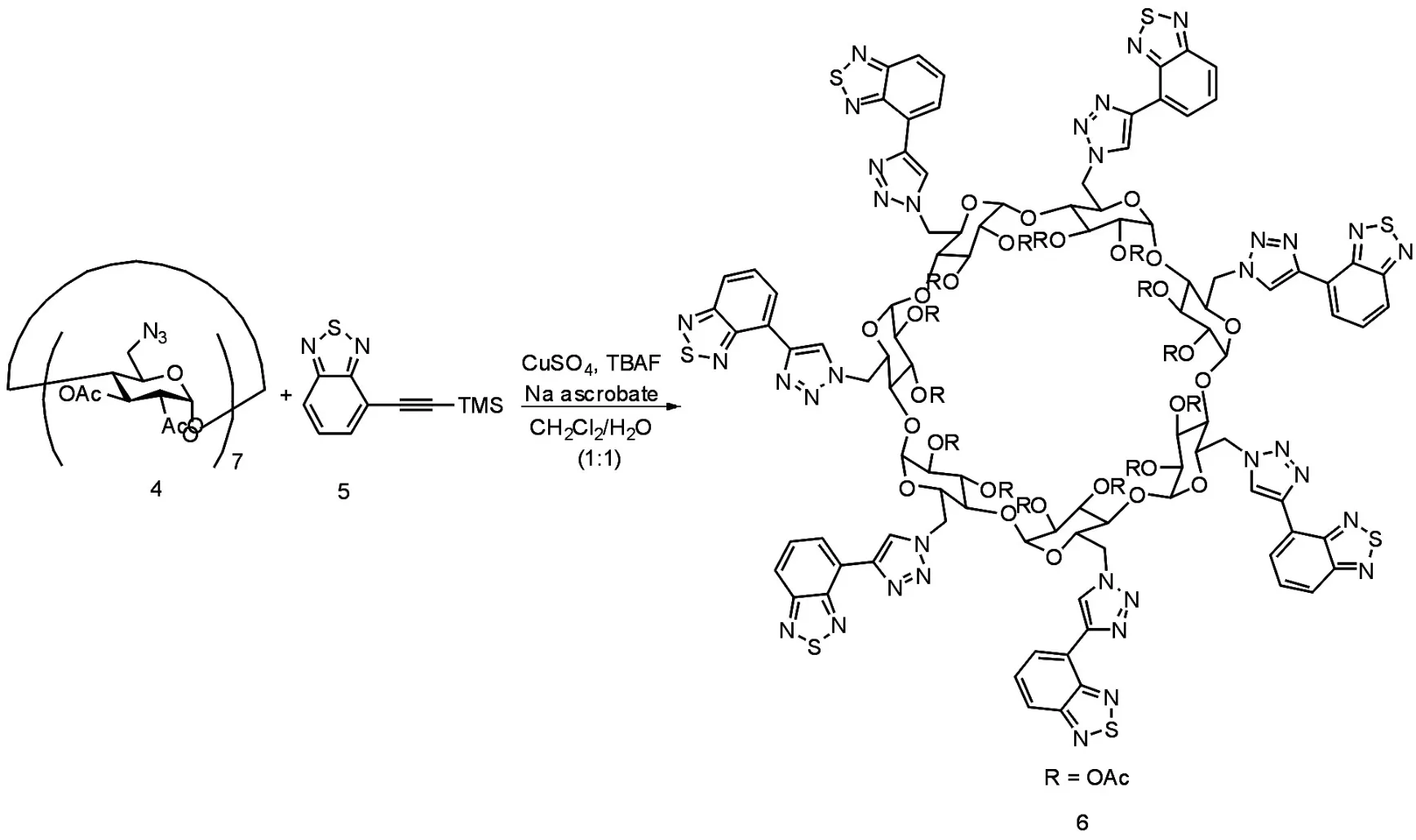
Scheme 1Synthesis of b-cyclodextrin substituted with benzothiadiazoyl-triazole moiety 6

Fig.4Structure of benzothiadiazolyl-bis-triazoyl amino acid derivative 7

Fig.5Structure of triazolyl coumarin derivatives 8-11
D Maity,et al[33]have synthesized another type of coumarin by"click"chemistry.This conformation⁃ally constrained pyrrolidinyl-triazolyl-bipyridyl cou⁃marin 13 serves as a selective chemosensor for Al3+in"off-on"type response in MeCN(Fig.6)13 exhib⁃its an absorption band centered around 301 nm.Ad⁃dition of Al3+resulted in a red-shift to 324 nm and a large enhancement of the emission intensity posi⁃tioned around 443 nm.The triazole ring together with the bipyridyl moiety and one of the carbonyl group serve as an ionophore for coordinating the cation.The coumarin moiety plays a dual role as both a reporting unit and a cation binding site.
Coumarin can be used as chemodosimeter for detection of Cu(II)by fluorogenic"click"reaction (Scheme 3)[34].The nonfluorescent azido coumarin 15 readily reacts with phenylacetylene 16 in the pres⁃ence of Cu(II)and sodium ascorbate(1mM)in v(DMSO)∶v(H2O)(1∶1)to afford a highly fluores⁃cent triazole product 17(with an emission band cen⁃tered around 460 nm).
The scope of the system was extended by in⁃cluding EDTA,which inhibited reduction of Cu2+and prevented the"click"reaction from occurring. Subsequent addition of Zn2+,Cd2+and Ca2+displaced Cu2+from EDTA,allowing the"click"reaction to pro⁃ceed.So this system can be used to sense Cu2+and using the same EDTA-mediated transduction,to sense Zn2+,Cd2+and Ca2+.

Scheme 2Possible binding mode of 10 with Hg(ClO4)2
2 Anion Recognition
As is the case for cations,anions recoginition has received considerable attention due to the impor⁃tant roles they play in biology and the environment.Generally,receptor interations with anions rely on Coulombic forces[35-37],hydrogen bonding[38-40],ion pairs[41],π-stacking interactions[42-43],chemical reac⁃tion or through coordinative interactions with metal ions incorporated into the structure[44].

Fig.6Binding mode of 13 with Al3+

Scheme 3"Click"reaction to create fluorescent compound
Molina and co-workers[45]have developed a tri⁃azole tethered ferrocene-pyrene dye 18 which has the ability of detecting pyrophosphate both by fluo⁃rescence and electrochemisty.The weak fluores⁃cence emission of 18,typical of a pyrene monomer, increase significantly in presence of pyrophosphate as a consequence of excimer formation in 19(an ex⁃cimer is defined as the dimer formed by interaction between an excited fluorophore and an identical fluo⁃rophore in the ground state resulting in a new struc⁃tureless emission located at longer wavelength than that of the monomer).This probe was associated with a cathodic shift of the ferrocene/ferrocenium redox couple,demonstrating the dual modality of anion sensing(Fig.7).
J S Kim,et al[46]reported a fluorescent sensor 20(Fig.8),bearing two methyl esters and two pyrenetriazole groups in opposite dispositions.It was shown to be selective for the binding of I-.20 displays mono⁃mer and excimer bands at 395 and 475 nm,respec⁃tively.Upon addition of I-,the pyrene monomer emis⁃sion increased while the excimer emission declined in a ratiometric manner.This ratiometric change is probably due to the encapsulation of I-in the cavity, delineated by the ester and triazole functionality,to form a 1∶1 complex as confirmed by mass spectrome⁃try.Thisencapsulationinducesconformational changes of the frame which removes one pyrene resi⁃due from the other.

Fig.7Binding mode of ferrocene-pyrene dye with HP2
Another chemosensor 21 containing the bis-tri⁃azole motif was prepared in an effort to create cavi⁃tandsandnanotubesfromcalix[4]arenesusing "click"chemistrybySantoyo-Gonzalezand co-workers(Fig.9)[47].Incorporation of an anthra⁃cene fluorophore as a lower rim cap afforded a cavi⁃tand that binds Br-and I-in methanol,resulting in a small increase of fl uorescence emission at 407 nm.

Fig.8Structure of pyrenyl-appended triazole-based calix[4]arene 20
3 CombinedAnionandCationRecognition
The number of reports of triazole-containing combined anion and cation sensors is very limited. X L Ni,et al[48]have developed a ratiometric fluores⁃cent receptor with a C3symmetric structure based on a pyrene-linked triazole-modi fi ed homooxacalix[3]-arene 22(Fig.10).This system exhibited an interest⁃ing ratiometric detection signal output for targeting cations and anions through switching the excimer emission of pyrene from the"on-off"to the"off-on" type in neutral solution.The fluorescence intensity (485 nm)of 22 gradually decreased("on-off")and was accompanied by enhancement of the emission (396 nm)as the concentration of Zn2+increased in v(CH3CN)∶v(CH2Cl2)(1 000∶1).Addition of the H2PO4-anion(40 equiv.)to the Zn2+complex of 22 re⁃sulted in a 67%increase in intensity(485 nm)in the presence of small amounts of water and in organic media(Scheme 4)[48].1H-NMR titration results sug⁃gested that the Zn2+center of receptor L·Zn2+(23) provided an excellent pathway of organizing anion binding groups for optimal host-guest interactions to form 24(Scheme 5)[48].

Fig.9Structure of calix[4]arenes-based macrocycle 21

Fig.10Structure of pyrene-linked triazole-modi fi ed homooxacalix[3]arene 22

Scheme 4Ratiometric fluorescent receptors for both Zn2+and H2Pions
Inanextensionoftheirworkoncy⁃clam-basedzincsensors[49-50],MHToddand co-workers prepared a novel cyclam-based fluores⁃cent sensor 25(Scheme 6)which can distinguish be⁃ tween Cu2+and Hg2+via selective anion-induced demetallation.This sensor is highly responsive to both Cu2+and Hg2+in neutral aqueous solution, which caused a significant quenching in fluores⁃cence emission(390 nm).Addition of specific anions such as I-and S2O32-causes a complete revival of flu⁃orescence only in the case of Hg2+,providing a sim⁃ple and effective method for distinguishing solutions containing Cu2+,Hg2+or a mixture of both ions,even in doped seawater samples.X-ray crystal structures of both the Hg2+sensor complex and a model Cu2+complex show that pendant triazole coordination oc⁃curs through the central nitrogen atom(N2)(Scheme 6).This is the first reported example of this unusual coordination mode in macrocycles.Fluorescence, mass spectrometry and1H-NMR experiments reveal that the mechanism of anion-induced fluorescencerevival involves either displacement of pendant coor⁃dination or complete removal of the Hg2+from the macrocycle,depending on the anion(Scheme 7)[51].

Scheme 5Proposed structure of 23·Zn2+for H2Panion complexion

Scheme 6Proposed structure of 25·M2+

Scheme 7Two possible mechanisms for anion-induced fluorescence revival
4 Conclusions and Future Perspectives
Fluorescent molecules,because of their special photophysical properties such as steady-state inten⁃sity,Stokes shift,quantum yield,life-time,reso⁃nance energy transfer and fluorophore brightness, have powerful applications in biological and analyti⁃cal sciences.Moreover,CuAAC reaction,has provided us with great convenience in organic synthesis.The resultant 1,4-disubstituted 1,2,3-triazole group has been demonstrated to be very versatile moiety in fluo⁃rescentchemosensor.Asshownbythisshort literature overview,combination of fluorescent mole⁃cules and CuAAC reaction has powerful applications in ion recognition.The fluorescent chemosensor and chemodosimeters not only can be used in ion recogni⁃tion but also can be used in many other field such as biology,medicalscience,materialchemistryandsoon.
[1]VALEUR B,LERAY I.Design principles of fluores⁃cent molecular sensors for cation recognition[J].Coord Chem Rev,2000,205(1):3-40.
[2]GELLMAN S H.Introduction:molecular recognition[J].Chem Rev,1997,97(5):1231-1232.
[3]YOON S,MILLER E W,HE Q,et al.A bright and specific fluorescent sensor for mercury in water,cells,and tissue[J].Angew Chem Int Ed,2007,46(35):6658-6661.
[4]AHAMED B N,GHOSH P.Selective colorimetric and fluorometric sensing of Cu(II)by iminocoumarin deriva⁃tive in aqueous buffer[J].Dalton Trans,2011,40(24):6411-6419.
[5]YUAN L,LIN W Y,CHEN B,et al.Development of FRET-based ratiometric fluorescent Cu2+chemodosime⁃ters and the applications for living cell imaging[J].Org Lett,2012,14(2):432-435.
[6]KARUPPANNAN S,CHAMBRON J C.Supramolecu⁃lar chemical sensors based on pyrene monomer–ex⁃cimer dual luminescence[J].Chem Asian J,2011,6(4):964-984.
[7]SAMB I,BELL J,TOULLEC P Y,et al.Fluorescent phosphane selenide as efficient mercury chemodosime⁃ter[J].Org Lett,2011,13(5):1182-1185.
[8]DU J J,HU M M,FAN J L,et al.Fluorescent chemo⁃dosimeters using"mild"chemical events for the detec⁃tion of small anions and cations in biological and environmental media[J].ChemSoc Rev,2012,41(12):4511-4535.
[9]DUONG T Q,KIM J S.Fluoro-and chromogenic che⁃modosimeters for heavy metal ion detection in solution and biospecimens[J].Chem Rev,2010,110(10):6280-6301.
[10]ANDO S,KOIDE K.Development and applications of fluorogenic probes for mercury(II)based on vinyl ether oxymercuration[J].J Am Chem Soc,2011,133(8):2556-2566.
[11]HUANG S,CLARK R J,ZHU L.Highly sensitive fluo⁃rescent probes for zinc ion based on triazolyl-contain⁃ing tetradentate coordination motifs[J].Org Lett,2007,9(24):4999-5002.
[12]MAISONNEUVE S,FANG Q,XIE J.Benzothiadiazo⁃yl-triazoyl cyclodextrin:a selective fluoroionophore for Ni(II)[J].Tetrahedron,2008,64(37):8716-8720.
[13]GARCIA L,MAISONNEUVE S,XIE J,et al.Sugars to control ligand shape in metal complexes:conforma⁃tionally constrained glycoligands with a predetermina⁃ tion of stereochemistry and a structural control[J].In⁃org Chem,2010,49(16):7282-7288.
[14]MAITY D,GOVINDARAJU T.Pyrrolidine constrained bipyridyl-dansyl click fluoroionophore as selective Al3+sensor[J].Chem Commun,2010,46(25):4499-4501.
[15]GARCIA L,MAISONNEUVE S,OUDINET-SIN MAR⁃CU J,et al.Intrinsically fluorescent glycoligands to study metal selectivity[J].Inorg Chem,2011,50(22):11353-11362.
[16]JIN Z,ZHANG X B,XIE D X,et al.Clicking fluo⁃roionophores onto mesoporous silicas:a universal strat⁃egy toward efficient fluorescent surface sensors for met⁃al ions[J].Anal Chem,2010,82(15):6343-6346.
[17]WU Y Z,DONG Y,LI J F,et al.A highly selective and sensitive polymer-based fluorescence sensor for hg2+-ion detection via click reaction[J].Chem Asian J,2011,6(10):2725-2729.
[18]CHANG K C,SU I H,LEE G H,et al.Triazole-and azo-coupled calix[4]arene as a highly sensitive chro⁃mogenic sensor for Ca2+and Pb2+ions[J].Tetrahedron Lett,2007,48(41):7274-7278.
[19]LIU Z,HE W J,GUO Z J.Metal coordination in photo⁃luminescent sensing[J].Chem Soc Rev,2013,42(4):1568-1600.
[20]ER J C,TANG M K,CHIA C G,et al.MegaStokes BODIPY-triazoles as environmentally sensitive turn-on fluorescentdyes[J].ChemSci,2013,4(5):2168-2176.
[21]LOUDET A,BURGESS K.BODIPY dyes and their de⁃rivatives:syntheses and spectroscopic properties[J]. Chem Rev,2007,107(11):4891-4932.
[22]URICH G,ZIESSEL R,HARRIMAN A.The chemistry of fluorescent bodipy dyes:versatility unsurpassed[J]. Angew Chem Int Ed,2008,47(7):1184-1201.
[23]VEDAMALAI M,WU S P.A BODIPY-based colori⁃metric and fluorometric chemosensor for Hg(II)ions and its application to living cell imaging[J].Org Bio⁃mol Chem,2012,10(28):5410-5416.
[24]SHI W J,LIU J Y,NG D K P.A highly selective colori⁃metric and fluorescent probe for Cu2+and Hg2+ions based on a distyryl BODIPY with two bis(1,2,3-tri⁃azole)amino receptors[J].Chem Asian J,2012,7(1):196-200.
[25]DA CRUZ EDUARDO H G,CARVALHO PEDRO H P R,CORRÊA JOSÉ R,et al.Design,synthesis and application of fluorescent 2,1,3-benzothiadiazole-tri⁃azole-linked biologically active lapachone derivatives[J].New J Chem,2014,38,2569-2580.
[26]NETO B A D,LOPES A S,EBELING G,et al.Photo⁃physical and electrochemical properties of π-extended molecular 2,1,3-benzothiadiazoles[J].Tetrahedron,2005,61(46):10975-10982.
[27]NETO B A D,LAPIS A A M,DA SILVA JUNIOR E N,et al.2,1,3-benzothiadiazole and derivatives:syn⁃thesis,properties,reactions,and applications in light technology of small molecules[J].Eur J Org Chem,2013,2013(2):228-225.
[28]RUAN Y B,MAISONNEUVE S,LI C,et al.Coopera⁃tive recognition of Cu2+based on amino acids tethered benzothiadiazoyl-bistriazoles[J].Front Chem China,2010,5(2):208-213.
[29]VASYLEVSKA A S,KARASYOV A A,BORISOV S M,et al.Novel coumarin-based fluorescent pH indica⁃tors,probes and membranes covering a broad pH range[J].Anal Bioanal Chem,2007,387(6):2131-2141.
[30]ALBERS A E,OKREGLAK V S,CHANG C J.A FRET-based approach to ratiometric fluorescence de⁃tection of hydrogen peroxide[J].J Am Chem Soc,2006,128(30):9640-9641.
[31]SOH N.Recent advances in fluorescent probes for the detection of reactive oxygen species[J].Anal Bioanal Chem,2006,386(3):532-543.
[32]HO I T,LAI T L,WU R T,et al.Design and synthesis of triazolyl coumarins as Hg2+selective fluorescent che⁃mosensors[J].Analyst,2012,137(24):5770-5776.
[33]MAITY D,GOVINDARAJU T.Conformationally con⁃strained(coumarin-triazolyl-bipyridyl)click fluoroion⁃ophore as a selective Al3+sensor[J].Inorg Chem,2010,49(16):7229-7231.
[34]VARAZO K,LE DROUMAGUET C,FULLARD K,et al.Metal ion detection using a fluorogenic"click"reac⁃tion[J].Tetrahedron Lett,2009,50(50):7032-7034.
[35]VANCE D H,CZARNIK A W.Real-time assay of inor⁃ganic pyrophosphatase using a high-affinity chela⁃tion-enhanced fluorescence chemosensor[J].J Am Chem Soc,1994,116(20):9397-9398.
[36]NIIKURA K,METZGER A,ANSLYN E V.Chemosen⁃sor ensemble with selectivity for inositol-trisphosphate[J].J Am Chem Soc,1998,120(33):8533-8534.
[37]CARCIA-ESPANA E,DIAZ P J,LLINARES M,et al. Anion coordination chemistry in aqueous solution of polyammonium receptors[J].Coord Chem Rev,2006,250(23/24):2952-2986.
[38]ANZENBACHER P,JURSIKOVA K J,SESSLER J L. Second generation calixpyrrole anion sensors[J].J Am Chem Soc,2000,122(38):9350-9351.
[39]BOWMAN-JAMES K.Alfred werner revisited:the co⁃ordination chemistry of anions[J].Acc Chem Res, 2005,38(8):671-678.
[40]LIN C,SIMOV V,DRUECKHAMMER D G.Interac⁃tion of halide and carboxylate Ions with 4,5-diacetami⁃doacridine-9(10H)-one:thermodynamics of associa⁃tion and deprotonation events[J].J Org Chem,2007,72(5):1742-1746.
[40]KIM S K,SESSLER J L.Ion pair receptors[J].Chem Soc Rev,2010,39(10):3784-3809.
[42]CHO H K,LEE D H,HONG J I.A fluorescent pyro⁃phosphate sensor via excimer formation in water[J]. Chem Commun,2005,13:1690-1692.
[43]LEE H N,XU Z,KIM S K,et al.Pyrophosphate-selec⁃tive fluorescent chemosensor at physiological pH:for⁃mation of a unique excimer upon addition of pyrophos⁃phate[J].JAmChemSoc,2007,129(13):3828-3829.
[44]STEED J W.Coordination and organometallic com⁃pounds as anion receptors and sensors[J].Chem Soc Rev,2009,38(2):506-519.
[45]ROMERO T,CABALLERO A,TARRAGA A,et al.A click-generatedtriazoletetheredferrocene-pyrene dyad for dual-mode recognition of the pyrophosphate anion[J].Org Lett,2009,11(15):3466-3469.
[46]KIM J S,PARK S Y,KIM S H,et al.A pyrenyl-ap⁃pended triazole-based calix[4]arene as a fluorescent sensor for iodide ion[J].Bull Korean Chem Soc,2010,31(3):624-629.
[47]MORALES-SANFRUTOS J,ORTEGA-MUNOZ M,LOPEZ-JARAMILLO J,et al.Synthesis of calix⁃arene-based cavitands and nanotubes by click chemis⁃try[J].J Org Chem,2008,73(19):7768-7771.
[48]NI X L,ZENG X,REDSHAW C,et al.Ratiometric flu⁃orescent receptors for both Zn2+and H2PO4-ions based on a pyrenyl-linked triazole-modified homooxacalix[3]arene:a potential molecular traffic signal with an R-S latch logic circuit[J].J Org Chem,2011,76(14):5696-5702.
[49]TAMANINI E,KATEWA A,SEDGER L M,et al.Pro⁃duction of no-carrier-added117mSn from proton irradiat⁃ed antimony[J].Inorg Chem,2009,48(2):319-324.
[50]TAMANINI E,FLAVIN K,MOTEVALLI M,et al. Conformationallyconstrained(coumarin-triazolyl-bi⁃pyridyl)click fluoroionophore as a selective Al3+Sensor[J].Inorg Chem,2010,49(16):3789-3800.
[51]LAU Y H,PRICE R J,TODD M H,et al.A click fluo⁃rophore sensor that can distinguish CuⅡand HgⅡvia selective anion-induced demetallation[J].Chem Eur J,2011,17(10):2850-2858.
(责任编辑:陈旷)
Recent Progress on Fluorescent Chemosensors and Chemodosimeters for Ion Recognition
YU Yanhua,FU Cheng
(Key Laboratory of Optoelectronic Chemical Materials and Devices of Ministry of Education,Institute for Interdisciplinary Research,Jianghan University,Wuhan 430056,Hubei,China)
Fluorescence detection is highly sensitive,and avoids the expense and difficulties of handling radioac⁃tive tracers for most chemical measurements.The definition and principle of chemosensors and chemodosimeters have been introduced.The application of chemosensors and chemodosimeters which were synthesized by Cu(I)-cat⁃alyzed Huisgen 1,3-dipolar cycloaddition of azides and terminal alkynes(CuAAC)reaction for ion recognition were reviewed from three parts:cation recognition,anion recognition and combined anion and cation recognition by re⁃cently reported examples.The fluorophores for cation detection were focused on 4,4-difluoro-4-bora-3a,4a-dia⁃za-s-indacene(BODIPY),benzothiadiazole and coumarin.
2014-01-20
O641
A
1673-0143(2014)03-0013-10
Biographies:YU Yanhua(1985—),female,assistant research fellow,majors in organic chemistry.
