功能化微纳米硅胶表面亚铁配位化学及其催化降解邻苯二酚的性质
2013-10-17魏振宏刘文明刘小明
刘 建 魏振宏 钟 伟 刘文明 刘 笑 刘小明*,,
(1南昌大学化学系,南昌 330031)
(2嘉兴学院生物与化学工程学院,嘉兴 314001)
0 Introduction
Iron is one of the most abundant elements in the earth crust and consequently one of the most favoured elements adopted by nature in the course of evolutions of “devising” enzymes to achieve specific functions required by a variety of life forms.Indeed,ironcontaining metalloenzymes are a large category of biocatalysts which are responsible for a wide range of catalytic functions,from the activation of C-H bond to degradation of aromatic molecules[1-4].Mononuclear nonheme iron-containing enzymes are one of the subgroups of a large enzymatic family,for example,protocatechuate 3,4-dioxygenase (3,4-PCD), 2,3-dihydroxybiphenyl 1,2-dioxygenase (BphC).In these metalloenzymes,the iron centre is coordinated by number of essential biological ligands provided by surrounding amino acid residues in addition to one or two loosely bound small molecules,for example,water and anions (such as carbonate,acetate etc.)where usually a substrate binds.The metal centre under the coordination of these ligands sits in a highly confined environment composed by its surrounding protein backbones. The highly confined coordination atmosphere renders the metal centre less saturated coordination compared to its behaviours in solution chemistry.The protein domains show also certain flexibility in geometric conformations upon substrate binding.These structural features ensure the natural metalloenzymes high efficiency and selectivity in catalysis,and particularly capability of activating recalcitrant substrates, for instance, methane oxidation.The incredible capabilities ofnatural systems have greatly inspired synthetic chemists to mimic the natural systems although precisely duplicating the features is extremely challenging,particularly in solution chemistry.
Enzymatic catalysis is intrinsically homogenous.In practical applications,such a process has difficulties in catalyst-separation and thus catalystrecycling although high catalytic efficiency is an advantage. Therefore, developing a strategy in mimicking natural enzymes is highly desirable to ensure catalyst-recovery and-recycling via simple filtration or centrifugation.Such a strategy should compromise at the least loss of the catalytic efficiency.To this end,immobilising homogenous catalyst onto a solid matrix is an option.A variety of materials can be employedforsuchapurpose[5-12].Among those materials,micro/nano silica gel(NSiG)have a particular interest in this regard to chemists owing to a number of advantageous properties,for example,high specific area,appropriate particle size which allows forming stable suspension,more or less behaving as a“true solution”,feasibility for surface modification,and wide availability with low cost.Because of these advantages,NSiG has been found applications in many areas as a favourable matrix,for instance,drug delivery[13-14],fluorescent labelling[15-16],adsorption[17-19],and catalysis[20-22].NSiG functionalised by organic moieties loading with or without transition metals has been able to achieve a variety of catalytic functionalities[5,23-24].
The instauration in coordination around a metal centre is crucial for the catalyst to function.In metalloenzymes,the instauration is partially achieved,for instance,by loosely bound water molecule as mentioned above.Owing to steric reason,this essential feature could be achieved for the metal centre loaded at the surface of functionalised NSiG if an appropriate functional moiety with multidentate ligating atoms is chemically grafted.
Herein,we report the functionalisation of NSiG with a bidentate ligand possessing “NS”-donor-set analogous to the ligand L (2-(ethylthiomethyl)pyridine).The functionalised silica gel,L-NSiG,was further reacted with Feギ,and the system,ironギ-complex@SiO2,was examined as a catalyst in catalytic degradation of catechol by hydrogen peroxide.This chemistry is also inspired generally by mononuclear nonheme ironギ enzyme,forexample,catechol dioxygenases of extradiol dioxygenase. All the materials were characterised by FTIR,SEM,and TGA.In the metallation,both ferrous chloride and sulphate were employed to examine the influence of the coordination chemistry of the metal ion on the silica gel on the catalysis.To shed light on the possible coordination mode of the iron centre anchored at the surface of the functionalised NSiG and therefore,its correlation to its catalytic behaviours,ligand L was employed to react with the precursor,FeCl2,to synthesise a control monoiron complex whose structure was confirmed using X-ray single crystal diffraction analysis.Its catalysis on the degradation of catechol by hydrogen peroxide was also examined.Our results indicate that catalytic efficiency is compromised when the catalyst is immobilised on the surface of the NSiG.But the negative influence can be offset by tuning the coordination mode via altering the auxiliary ligand around the Feギ centre.
1 Experimental
1.1 General procedures
Reactions involved in this work were routinely performed and Schlenk technique was employed when necessary.All chemicals were purchased from local suppliers unless otherwise stated.Organic solvents were freshly distilled prior to use with appropriate drying agents.FTIR spectral data (KBr pellets)were collected in the range of 400~4 000 cm-1on VARIAN 2000 FT-IR.UV-Vis spectra were recorded in the range of 200~800 nm on a VARIAN 50 Conc.The particle diameter of silica gel was determined on a Quanta 200F (FEI,Netherlands)in the SEM mode.Thermal gravimetric analysis of the functionalised NSiG was performed on a TA SDTQ600 under N2flow at a heating rate of 10 ℃·min-1.
2-(Bromomethyl)pyridine (1·HCl)was prepared using literature procedure[25].Sub-micro silica gel(100~250 nm)and 3-chloropropyltriethoxysilane were locally purchased (Qingdao Ocean Scientific Co.,Ltd.China and Novel Materials of Organosilicone Co.,Ltd.of Wuhan University,China,respectively,http://www.wdsilicone.cn/).2-methylpyridine and ethanethiol were purchased from Sigma-Aldrich and used as received.Unless otherwise stated, solvents for flash chromatography were purchased from local suppliers.
Crystallographic data of complex[Fe(L)2Cl2]were collected on a Bruker SMART CCD diffractometer with graphite-monochromated Mo Kα radiation (λ=0.071 073 nm).The crystal structure was solved using direct methods in SHELXS and refined by full-matrix least-squares routines,based on F2,using the SHELXL package[26].
The catalytic activities of complex[Fe(L)2Cl2]and the functionalised silica gel,FeC@L-NSiG and FeS@LNSiG,in catechol oxidation by hydrogen peroxide wereestimated usingHPLC (Agilent1200)by determining the remaining catechol in the sample after oxidative degradation.Typical procedure was as follows.Catechol(45 mg)was dissolved in H2O(50 mL).To this solution was added the catalyst(FeC@LNSiG(10 mg),FeS@L-NSiG(10 mg),or[Fe(L)2Cl2](2 mg))following the addition of H2O2(2 mL,30%,V/V).The control experiment was performed using the same procedure as described but without addition of any catalyst.The reaction mixture was stirred at 30oC for 160 min.To examine the remaining catechol,sampling for HPLC analysis was taken every 15 min.The high-performance liquid chromatography using a UV-Vis detector set at 270 nm.The stationary phase is a kromasil C-18 (4.6 mm ×250 mm),while the mobile phase is a mixture of methanol and water,50:50(V/V),at a flow rate of 1.0 mL·min-1.
1.2 Synthesis
Ligand L,2-(ethylthiomethyl)pyridine,compound 2 and the complex,[Fe(L)2Cl2]
2-(Ethylthiomethyl)pyridine (L):Sodium lumps(0.50 g,21.7 mmol)were dissolved in ethanol(20 mL)at ice-temperature, to which was then added ethanethiol(0.65 mL,7.2 mmol)under stirring for 1 h underargon atmosphere.Afterthereaction was warmed to room temperature,a solution of compound 1HCl(1.00 g,4.8 mmol)in ethanol(10 mL)was added through a pressure-equalized funnel.After the addition,the reaction was kept under stirring at room temperature for further 2 h.After any insoluble materials were filtered off,the obtained filtrate was evaporated by rotary evaporation to produce a residue which was dissolved in water(10 mL).The aqueous solution was extracted successively with ether(35 mL×3).All the extracts were combined before being dried with anhydrous Na2SO4.Removal of the solvents under reduced pressure gave a crude product which was further purified using flash chromatography on silica gel column (ethyl acetate∶petroleum ether=1∶4)to produce pale yellow and stinky oily liquid(536 mg,73%).1H NMR (600 MHz,CDCl3):8.53(m,1H,6-PyH),7.65(m,1H,4-PyH),7.38(d,J=7.8 Hz,1H,3-PyH),7.16(m,1H,5-PyH),3.85(s,2H,PyCH2),2.51(m,2H,SCH2),1.24(d,J=7.2 Hz,3H,CH3).
Pyridin-2-ylmethanethiol (2):2-(bromomethyl)pyridine hydrochloride(5.00 g,24 mmol)and thiourea(4.57 g,60 mmol)were dissolved in H2O(50 mL)in a round-bottom flask under argon.The solution was stirred for 3 h at 90℃.Then the reaction was degassed using Arflow while being cooled to an icetemperature.To the pre-cooled solution was added sodium hydroxide (5.30 g,0.13 mol)in four portions over the period of 2 h while maintaining the reaction at ice temperature.The solution was further stirred overnight at room temperature under Ar before it was extracted with diethyl ether(35 mL×3).The aqueous phase was collected and cooled to ice-temperature before its acidity was adjusted to pH=7 with concentrated hydrochloric acid.The neutralised aqueous phase was extracted with dichloromethane(15 mL×3).The organic phases were combined,washed twice with brine(20 mL,26.5%,m/m),and dried with anhydrous MgSO4before it was evaporated by rotary evaporation to produce a pungent oily liquid (1.50 g,50%).1HNMR(600 MHz,CDCl3):8.53(s,1H,6-PyH),7.64(m,1H,4-PyH),7.33 (d,J=7.8 Hz,1H,3-PyH),7.15(m,1H,5-PyH),3.84(d,J=7.2 Hz,2H,PyCH2),2.027(t,J=7.8 Hz,1H,SH).
[Fe(L)2Cl2]:FeCl2(100 mg,0.787 mmol)and ligand L (296 mg,1.934 mmol)were dissolved in methanol (6 mL)under argon and the mixture was stirred for 2 h at room temperature to give a yellow solid.The solid was collected via filtration,then washed with methanol(1 mL×3)and ether(3 mL×3),respectively, and finally dried under vacuum.Recrystallisation was performed by dissolving the solid in a minimum methanol layered with diethyl ether.Yellow crystals(247 mg,72%)suitable for X-ray single crystallographic analysis were collected in a few days at room temperature.Anal.Calcd.for[Fe(L)2Cl2](%):C,44.36;H,5.12;N,6.47.Found:C,44.02;H,4.87;N,6.43.
1.3 Functionalisation of NSiG
Preparation of Cl-NSiG:NSiG(3 g)was vacuumdried for 8 h at 160℃in Schelenk flask.To the flask was then successively added toluene(150 mL)and 3-chloropropyl-triethoxysilane(2.25 g,6.84 mmol)after being cooled to room temperature.The mixture was heated under reflux for 48 h.After the reaction mixture being cooled to room temperature,the silica gel was separated,and washed successively with toluene(20 mL×3),ethanol(20 mL×3)and diethyl ether(20 mL×3)to remove any unreacted 3-chloropropyltriethoxysilane.The collected off-white solid (2.87 g),Cl-NSiG,was dried under vacuum for 6 h at 80℃.Microanalysis(%):2.65(C),1.47(H),0.80(N).
Preparation of L-NSiG:Under argon,Cl-NSiG(2.0 g)was dispersed in toluene (100 mL).To the suspension was added compound 2 (1.20 g,9.60 mmol),K2CO3(1.12 g,8.11 mmol)and KI(0.67 g,4.04 mmol).The mixture was refluxed for 48 h at 120℃.After the reaction mixture being cooled to temperature,the silica gel was separated and washed successively with distilled water(20 mL×3),ethanol(20 mL×3),toluene (20 mL×3),and finally diethyl ether (20 mL×3)to remove any inorganic salts and unreacted compound 2.The off-white solid (1.67 g),L-NSiG,was dried under vacuum for 6 h at 80℃.Microanalysis(%):1.18(C),1.14(H),0.18(N).
Preparation of FeC@L-NSiG:FeCl2was employed as a precursor to load the functionalised silica gel(LNSiG)with Feギto achieve the functionalised silica gel,FeC@L-NSiG.Typical procedure was as follows.L-NSiG(300 mg)and FeCl2(100 mg)were dispersed in methanol (10 mL)under argon,and the mixture wasstirred for8 h atroom temperature.The metallated solid was collected by filtration,washed successively with distilled water degassed using argon,methanol and finally diethyl ether as described above before it was dried under vacuum for 6 h at 80℃.Microanalysis(%):2.18(C)1.23(H),0.21(N).FeS@L-NSiG was analogously achieved except for that ferrous sulphate was used to replace the ferrous chloride.Microanalysis(%):3.19(C),1.44(H),0.17(N).
1.4 Catalytic activity of functionalised silica gel loaded with Feギ
The catalytic activity of the functionalised silica gel after being loaded with Feギwas assessed using the degradation of catechol by H2O2in aqueous solution.In the assessment,the emphasis was placed on the conversion of catechol and the identification of the degradation products was not pursued.To assess the catalytic behaviours of the control complex and the two functionalised materials,unmodified silica gel(NSiG)was employed as a control.To ensure that the iron content of the silica gel-based was approximately equal to that of the control complex,TGA data were employed to justify the amount of the functionalised materials used in the catalysis assessment.
2 Results and discussion
2.1 Synthesis of ligand L,compound 2 and complex[Fe(L)2Cl2]
The bidentate ligand 2-(ethylthiomethyl)pyridine(L)containing pyridine N and thioether S ligating atoms was synthesised by following the literature procedures[27],Scheme 1.Its proton NMR data are in agreement with those reported[28-29].The 2-(bromomethyl)pyridine (1)was synthesised from the reaction of 2-(methyl)pyridine with NBS and AIBN under visible irradiation[25].The compound was kept in the form of chloride and was regenerated from using sodium ethoxide prior to use.From the same precursor,pyridin-2-ylmethanethiol(2)for use in functionalising NSiG (vide infra)was also prepared by following literature procedure[30],Scheme 1.
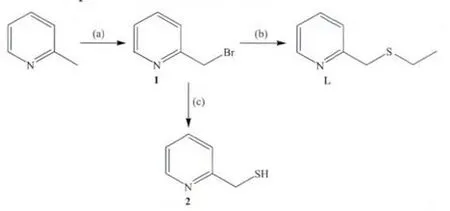
Scheme 1 Synthesis of ligands L(2-(ethylthiomethyl)pyridine)and 2(pyridin-2-ylmethanethiol):(a)NBS,AIBN/CCl4,(b)ethanethiol,CH3CH2ONa/CH3CH2OH,(c)thiourea,H2O and NaOH
Complex[Fe(L)2Cl2]was synthesised from the reaction of ligand L with FeCl2in methanol.Goldenyellow crystals were obtained from a solution of the complex in methanol into which diethyl ether was slowly diffused.Crystallographic details for complex[Fe(L)2Cl2]are shown in Table 1.Its structure and selected bond length and angles are listed in Fig.1 and Table 2,respectively.In the structure of complex[Fe(L)2Cl2],there is a symmetrical crystallographic centre locating at the Fe atom.The metal centre takes a slightly distorted octahedral geometry with the twopyridine nitrogen atoms and two thioether sulphur atoms from two ligands at the equatorial positions whereas the two Cl atoms reside at the axial positions.The three pairs of ligating atoms (N,S and Cl)are perfectly trans to each other.Analogous coordination geometryforFeギ wasfound in complex[Fe{(PyCH2SCH2)2CH2}Cl2][31].In complex[Fe(L)2Cl2],the values of Fe-N,Fe-S and Fe-Cl bond lengths are longer by ca.0.012 43,0.013 78 and 0.002 65 nm,respectively,than the corresponding bond lengths in complex[Fe(PyCH2SCH2)2CH2)Cl2]. This shortening may be attributed the chelating effectofthe tetradentate ligand(PyCH2SCH2)2CH2.
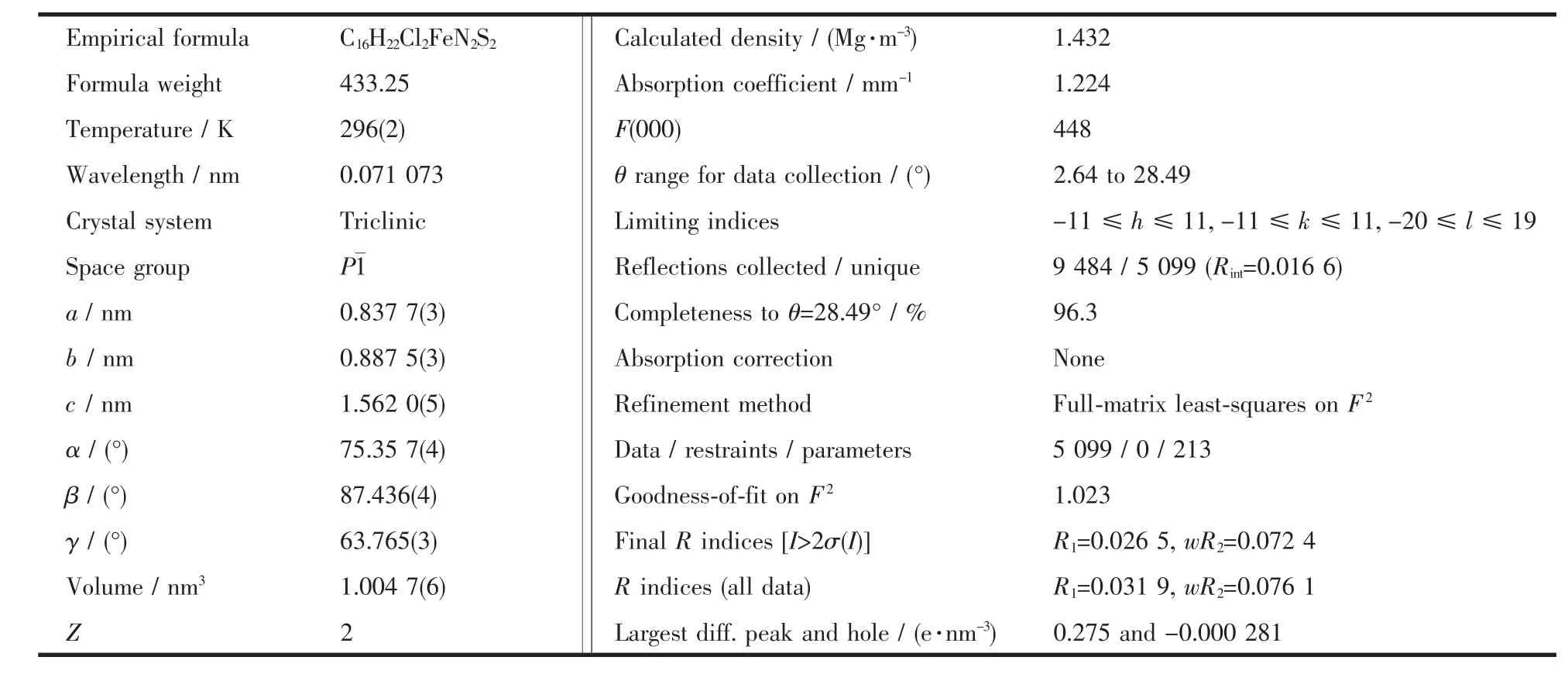
Table 1 Crystallographic details for complex[Fe(L)2Cl2]
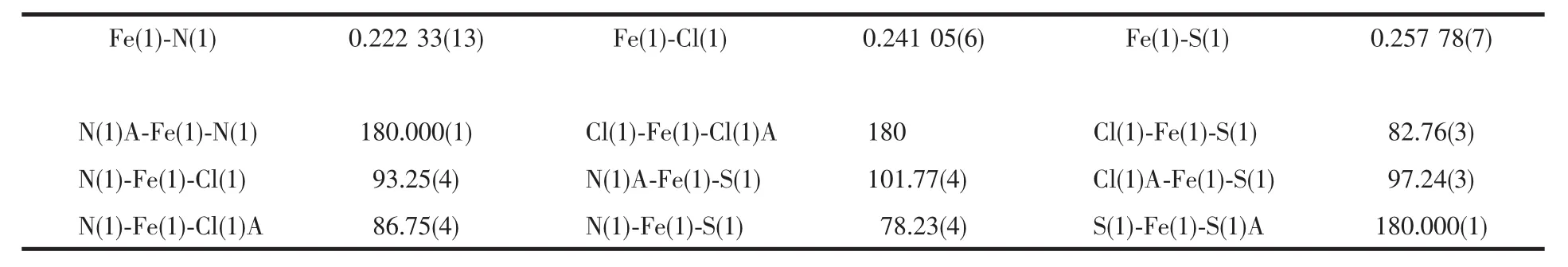
Table 2 Selected bond distances(nm)and angles(°)for[Fe(L)2Cl2]
CCDC:866526.
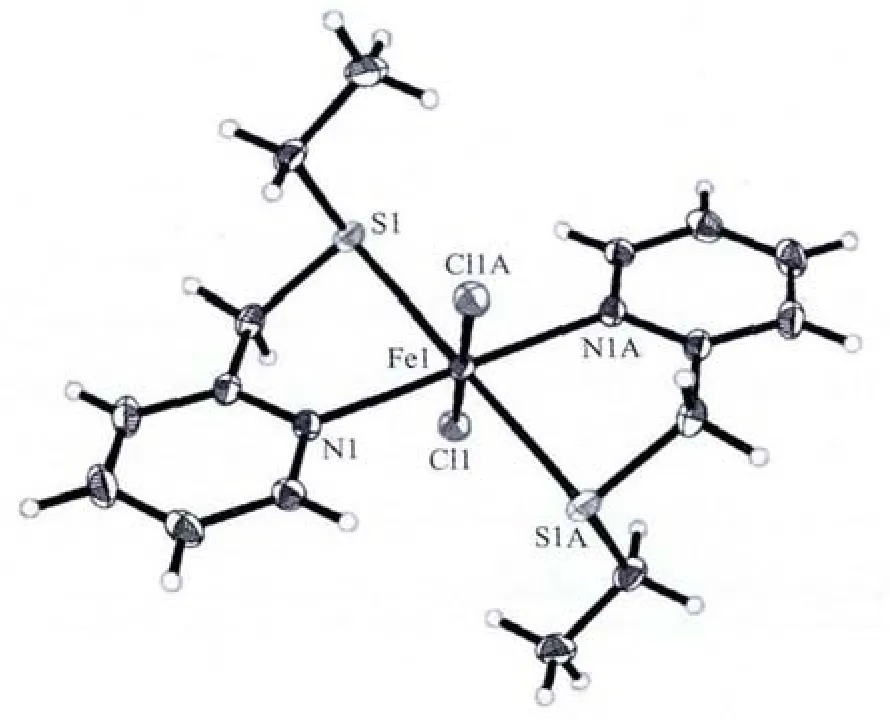
Fig.1 Crystal structure of complex[Fe(L)2Cl2]
2.2 Functionalisation of NSiG
To chemically graft compound 2 onto the surface of NSiG,the silica gel was first modified by reacting 3-chloropropyltriethoxysilane with silica gel[19].The introduction of chloropropyl group enables compound 2,the bidentate ligand for binding Feギ,to be anchored onto the surface of the silica gel to prepare the pre-modified silica gel,L-NSiG.Loading Feギonto the surface of L-NSiG was completed by stirring L-NSiG with FeCl2in methanol under room temperature to give the functionalised silica gel,FeC@L-NSiG,Scheme 2.Using FeSO4to metallate L-NSiG produced analogous material FeS@L-NSiG.The successful functionalisation was supported by microanalysis and infrared spectroscopic characterisation.
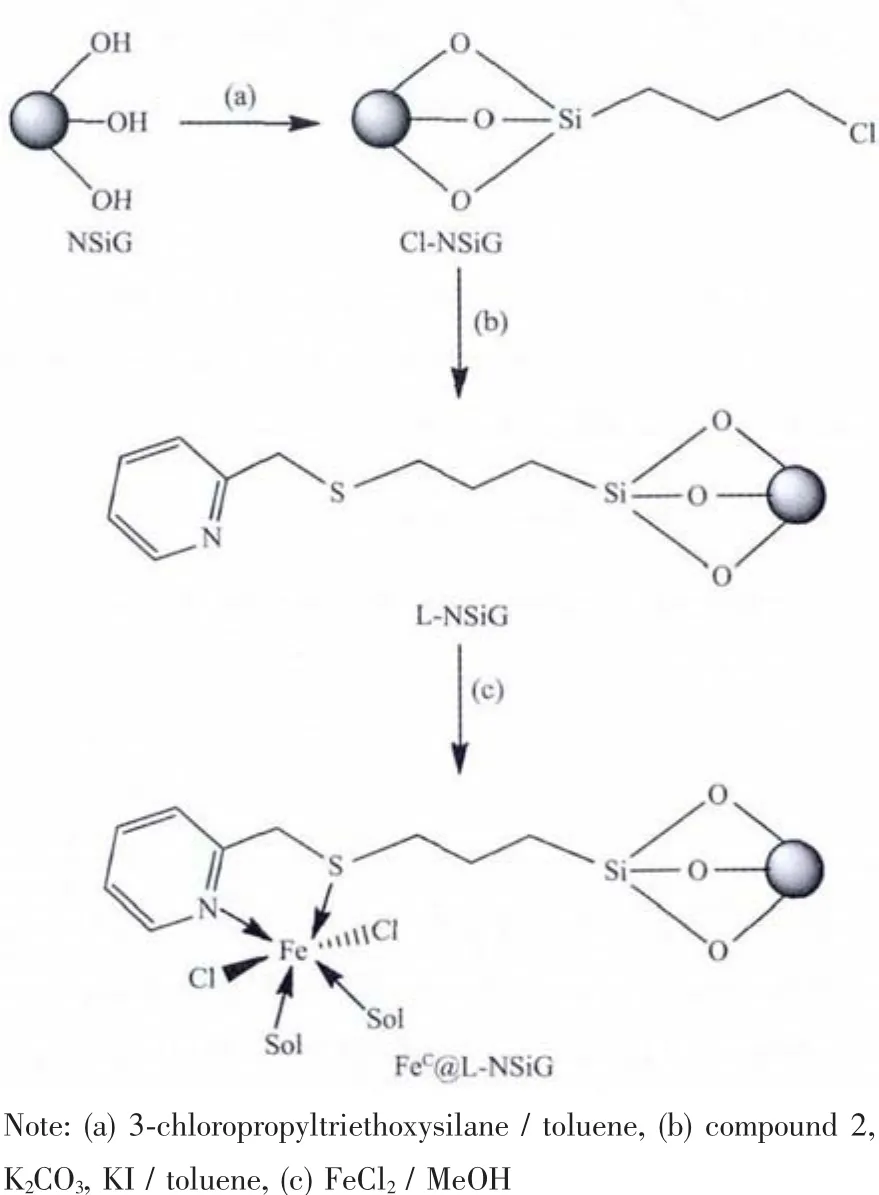
Scheme 2 Preparations of Cl-NSiG,L-NSiG,FeC@LNSiG and possible binding mode of Feギon the surface of the silica gel
Please note that FeS@L-NSiG ought to have similar coordination sphere to that of its chloride analogue as suggested by the UV-Vis spectra(Fig.4)with the two chlorides replaced with solvent molecules.
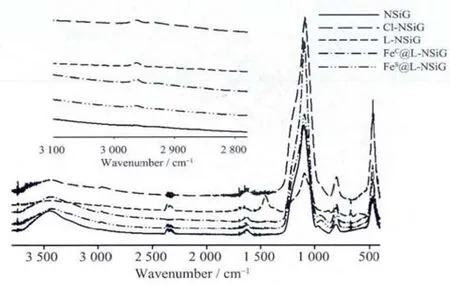
Fig.2 FTIR spectra for NSiG,L-NSiG,FeC@L-NSiG,and FeS@L-NSiG(KBr pellets)
The infrared spectra of the silica gel at various stages are shown in Fig.2.Apart from the absorption bands(ca.3 500 cm-1)arising from water and Si-O bond at ca.1 200 cm-1,there are other absorption bands indicating the successful immobilisation of organic groups on the surface of silica gel.The band at about 2 900 cm-1,which is absent in the spectrum of the silica gel(NSiG)before any modification,is a direct evidence of the immobilisation of organic moieties since this band is attributed to the stretching frequency of C-H bond from both CH2and CH3groups.Successful functionalisation of compound 2 is further confirmed by UV-Vis spectra in Fig.4.
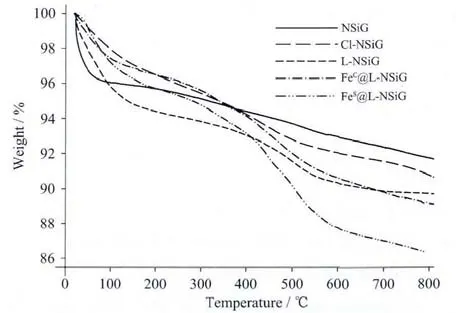
Fig.3 TGA curves for NSiG,Cl-NSiG,L-NSiG,FeC@LNSiG and FeS@L-NSiG
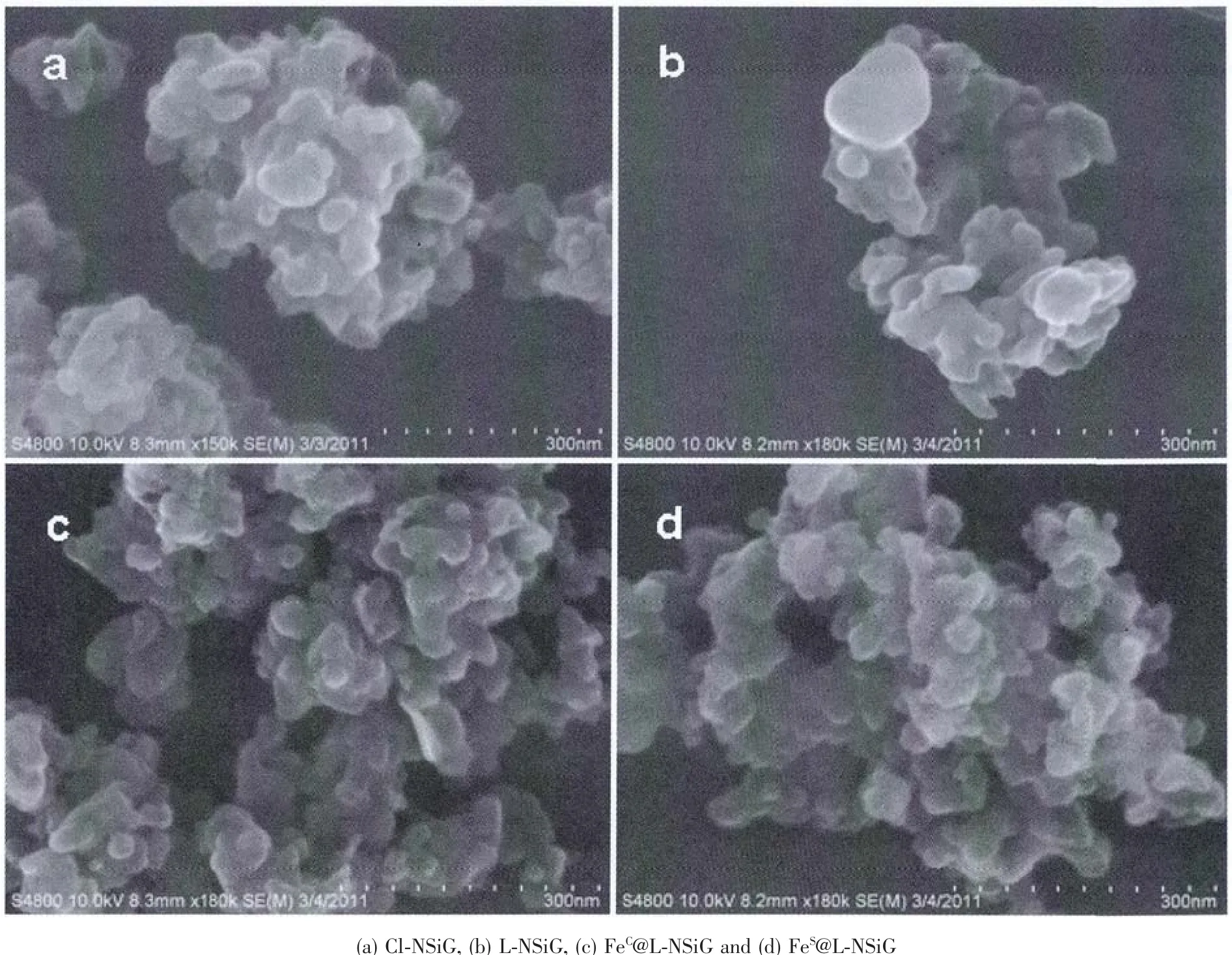
Fig.5 SEM images of the functionalised silica gel
The thermal stability ofthesilicagelwas assessed using thermal gravimetric analysis(TGA)in the range of 20~800 ℃.As shown in Fig.3,for the unmodified NSiG,only one major weight-loss(about 4%)was observed and attributed to the physically adsorbed water.After modifications,at least one more weight-loss approximately at 400℃was observed in addition to the water-loss.The second process accounts for about 5%weight-loss.Loading Feギonto the functionalised silica gel does not significantly alter the overall TGA trace as shown in Fig.3.The TGA traces show that the residue after thermal decomposition decreases steadily with the immobilisation of organic moiety onto the surface of the silica gel particles.The SEM images of silica gel at various stages are shown in Fig.5.These materials do not show substantially variations in their morphologies as revealed by the SEM images in Fig.5.It is also true that the diameters of the particles of these materials are comparable to that of the precursor.
2.3 Possible binding modes of Feギon the functionalised silica gel
As revealed by both FTIR and TGA data,Feギis readily loaded to the functionalised silica gel(LNSiG).This is certainly through coordination between the metal ion and the donating atoms (S and N).To probe the binding mode of Feギwith the binding sites on the surface of the silica gel,we employed ligand L(Scheme 1)which is analogous to the functional group with “SN”donor-set to react with FeCl2via solution chemistry.As shown in Fig.1 and Table 2,it is a binary complex with the two bound chloride ions perfectly trans to each other.Essentially,the overall structure is an octahedral geometry as pointed out earlier.
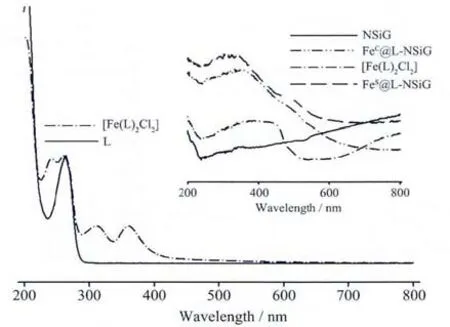
Fig.4 UV-Vis spectra of ligand L and complex[Fe(L)2Cl2]in CH3CN(inset:solid-state UV-Vis spectra of[Fe(L)2Cl2],NSiG,FeC@L-NSiG and FeS@L-NSiG)
But we need to bear in mind that the binding mode found in the solution chemistry ought to be rather different from that on the surface of L-NSiG due to steric effect caused by the constrain by the solid state of the organic moiety after immobilisation.Since pyridinyl group is a π-acceptor and metal-toligand charge transfer (MLCT)bands are expected when the low valent Feギis coordinated.Therefore,electronic spectra would be informative in probing the coordination chemistry of the metal ion. For comparison,the spectrum of the control complex is also shown in Fig.4.The MLCT bands found in the controlcomplexbetween 300 and 400 nm are analogously observed as a broad band in its solid state,Fig.4 (inset).The absorption of the control complex at the region below 600 nm is significantly different from that of the two materials.The electronic spectra in solid state indicate that,わthe metallation is successful since there is no any absorption bands observed for NSiG from 300 to 500 nm,which is also supported by Fe content from inductively coupled plasma (ICP)analysis results of FeC@L-NSiG and FeS@L-NSiG with 0.087 mmol·g-1and 0.10 mmol·g-1,respectively.ぷthe ironギon the surface of the functionalised silica gel shows different coordination chemistries from that of the control complex,[Fe(L)2Cl2].ぺrather similar chemistry is expected for both FeC@L-NSiG and FeS@L-NSiG since there are hardly differences observed except for a slight“red”shift by a few nanometres for the latter material.
Due to steric reason,the coordination of each metal ion on the functionalised silica gel could be satisfied by only one “NS” donor-set plus solvent(H2O)and anions,which is significantly different from the control complex as indicated by their UV-Vis spectra shown in Fig.4 (inset).For FeC@L-NSiG and FeS@L-NSiG,the most possible coordination sphere around the metal ion are “Fe(NS)Cl2(OH2)2”and “Fe(N,S)(X)4” (X=solvent or sulphate),respectively,if we assume that the metal ion requires six coordination number as well at the surface of the silica gel.But the binding of either the water molecule or the sulphate to Feギwould be rather weak.This would be advantageous in catalysis since those weakly bound molecules dissociate readily to provide vacant sites upon substrate binding. Indeed, the catalytic efficiency of the two materials in the degradation of catechol by hydrogen peroxide is significantly different(see section 2.4).
2.4 Catalytic assessment of the functionalisedsilica gel(FeC@L-NSiG and FeS@L-NSiG)
The conversion rates of catechol oxidation against the reaction coordinate are shown in Fig.6.As the catalytic data demonstrate,the monoiron complex shows both the fastest catalysis and the highest conversion rate.For the functionalised silica gel,FeS@L-NSiG,its conversion rate under the same conditions could be essentially comparable to that of the monoiron complex butits reaction rate is considerably slower.For FeC@L-NSiG,its conversion rate is one-fold lower compared to its analogue.
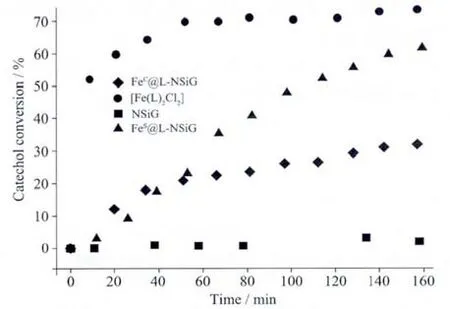
Fig.6 Oxidation of catechol by H2O2with the presence of FeC@L-NSiG,FeS@L-NSiG,[Fe(L)2Cl2]and NSiG
As heterogeneous catalysts,the reusability of FeC@L-NSiG and FeS@L-NSiG were investigated by recycling for three times.The conversion rate of catechol decreases from 31%to 2%for FeC@L-NSiG and from 61%to 10%for FeS@L-NSiG.To find out whether the efficiency-loss is due to losing the metal ion or the degradation of the systems during the catalysis,we added FeCl2to the recovered FeC@LNSiG isolated from the third cycle and performed the catalysis again under the same conditions.The nearly identical conversion rate to that obtained in the first round catalysis indicates that the catalyst is fully regenerated.This result suggests that the degradation of the systems caused by H2O2is neglectable if any.Indeed,when FeC@L-NSiG is treated with H2O2for 1 h,28% conversion rate is observed.This is in agreement with the above discussion that the decrease in catalytic efficiency in repetitive running is mainly caused by the loss of metal ion.
Since the catalysis of the monoiron complex is a homogeneous process,it is not surprising that the complex exhibits the fastest reaction rate and the highest catalytic performance.What are interesting are the catalytic behaviours of the two functionalised materials,FeC@L-NSiG and FeS@L-NSiG.Apparently,immobilising the ferrous ion onto the surface of the silica gel particles via coordination reduces considerably the rate ofcatalytic reaction.The immobilisation compromises the advantage possessed by the Feギcomplex in homogenous catalysis.As suggested by the electronic spectra of the two materials(inset in Fig.4),the coordination chemistry of the Feギon the surface of the two materials is essentially similar.As discussed earlier,the most possible difference between the two lies in the coordination sphere around the metal ion,“Fe(NS)Cl2(OH2)2” for FeC@L-NSiG and“Fe(N,S)(X)4” for FeS@L-NSiG(X=solvent or sulphate).Either the binding of H2O or sulphate (if any)is more labile compared to that of chloride.As mentioned earlier,this lability offers easier access for the binding of the substrate compared to FeC@L-NSiG.Therefore,the coordination sphere of the Feギwith more loosely bound ligands on the surface of FeS@L-NSiG is beneficial to the catalysis.Although electronic effect originated from the variation in coordination chemistry has also a role to play in affecting the catalysis,easy access for the binding of the substrate to the catalytic centre is certainly important as well in improving the catalysis.The results show that exploiting the steric effect possessed intrinsically by the solid material functionalised with organic functional groups is a feasible strategy to tune the catalytic property of the metal centre anchored onto the surface of micro/nano silica gel via coordination chemistry.
3 Conclusions
In summary,two functionalised materials,FeC@L-NSiG and FeS@L-NSiG,were prepared from micro/nano silica gel.The functionalisation is confirmed using FTIR andUV-Visspectroscopy,TGA andSEM techniques.On the surface of the materials are anchored organic functional groups analogous to a bidentate ligand L.Using complexes[Fe(L)2Cl2]as a control fully characterised using a variety of techniques,the coordination chemistry of the Feギon the surface of the two solid materials and their catalytic performance towards the oxidative degradation of catechol by hydrogen peroxide were explored. Although immobilising the catalytic centre onto the surface of the silica gel does compromise its catalytic efficiency,the functionalised silica gel materials have the advantages of easy separation and recycling.Furthermore,the steric effect could be exploited to tune the coordination chemistry around the metal centre and thus to improve the catalytic performance.Improvement in catalysis of the functionalised materials of this type could be further achieved by tuning the length of the linkage between the organic functional group and the silica gel particle,the steric and electronic effect of the organic moiety.Therefore,applying the coordination features found in metalloenzymes to functionalising solid materials such as micro/nano silica gel will be of great potentials in developing novel catalytic materials.Such materials would have high catalytic efficiency,easy separation,recycling and possibly improved selectivity.
Acknowledgements:We thank the National Natural Science Foundation of China(Grant Nos.20871064,21171073)for financial support and the provincial government of Zhejiang is also acknowledged for the Qianjiang Professorship (XL)at Jiaxing University.
[1]Costas M,Mehn M P,Jensen M P,et al.Chem.Rev.,2004,104(2):939-986
[2]Bugg T D H,Winfield C J.Nat.Prod.Rep.,1998,15(5):513-530
[3]Bugg T D H,Lin G.Chem.Commun.,2001:941-952
[4]Que L,Ho R Y N.Chem.Rev.,1996,96(7):2607-2624
[5]Maldotti A,Molinari A,Varani G,et al.J.Catal.,2002,209(1):210-216
[6]Marion R,Muthusamy G,Geneste F.J.Catal.,2012,286:266-272
[7]Zhou H,Wang Y M,Zhang W Z,et al.Green Chem.,2011,13(3):644-650
[8]Alvaro M,Baleizao C,Carbonell E,et al.Tetrahedron,2005,61(51):12131-12139
[9]Motokura K,Itagaki S,Iwasawa Y,et al.Green Chem.,2009,11(11):1876-1880
[10]Park D W,Yu B S,Jeong E S,et al.Catal.Today,2004,98(4):499-504
[11]Sun D,Zhai H,Catal.Commun.,2007,8(7):1027-1030
[12]Xiao L F,Li F W,Xia C G.Appl.Catal.A-Gen.,2005,279(1-2):125-129
[13]Huang S S,Fan Y,Cheng Z Y,et al.J.Phys.Chem.C,2009,113(5):1775-1784
[14]Klichko Y,Liong M,Choi E,et al.J.Amer.Cer.Soc.,2009,92(1):S2-S10
[15]Burns A,Ow H,Wiesner U.Chem.Soc.Rev.,2006,35(11):1028-1042
[16]Yao G,Wang L,Wu Y R,et al.Anal.Bioanal.Chem.,2006,385(3):518-524
[17]Arguello J,Leidens V L,Magosso H A,et al.Electrochim.Acta,2008,54(2):560-565
[18]Boddi K,Takatsy A,Szabo S,et al.J.Sep.Sci.,2009,32(2):295-308
[19]Peng Y,Li Z M,Zeng Y B,et al.Microchim.Acta,2010,170(1-2):17-26
[20]Hlatky G G.Chem.Rev.,2000,100(4):1347-1376
[21]Li K T,Dai C L,Kuo C W.Catal.Commun.,2007,8(8):1209-1213
[22]Jin Y H,Li A Z,Hazelton S G,et al.Coord.Chem.Rev.,2009,253(23-24):2998-3014
[23]Bigi F,Moroni L,Maggi R,et al.Chem.Commun.,2002:716-717
[24]Heravi M M,Sadjadi S,Oskooie H A,et al.Ultrason.Sonochem.,2009,16(6):718-720
[25]Ijuin R,Umezawa N,Higuchi T.Bioorgan.Med.Chem.,2006,14(10):3563-3570
[26]Sheldrick G.Acta Crystallogr.Sect.A,2008,64:112-122
[27]Reisner E,Abikoff T C,Lippard S J.Inorg.Chem.,2007,46(24):10229-10240
[28]van Bommel K J C,de Jong M R,Metselaar G A,et al.Chem.Eur.J.,2001,7(16):3603-3615
[29]Canovese L,Visentin F,Uguagliati P,et al.Inorg.Chim.Acta,1998,275-276:385-394
[30]Remuzon P,Bouzard D,Dicesare P,et al.Tetrahedron,1995,51(35):9657-9670
[31]Patra A,Sarkar S,Drew M G B,et al.Polyhedron,2009,28(7):1261-1264
