Why bortezomib cannot go with ‘green’?
2013-09-26LiJiaFengTingLiu
Li Jia, Feng-Ting Liu
1Center for Hemato-Oncology, Barts Cancer Institute, 2Division of Hemato-Oncology, St Bartholomew’s Hospital, Barts Health NHS Trust, Queen Mary University of London, London E14NS, UK
Introduction
Bortezomib (codenamed as PS-341 and marketed as Velcade by Millennium Pharmaceuticals) was developed as a potent,specific proteasome inhibitor for the treatment of relapsed and refractory multiple myeloma1and currently it is still one of the most effective drugs currently available for treating multiple myeloma.While bortezomib alone achieved a 40% response rate (RR), the RR was further improved to 88% in combination with dexamethasone2.However, only 4 of 15 patients with acute leukemia showed a decrease in blast count3.Bortezomib has also shown poor efficacy in the treatment of chronic lymphocytic leukemia (CLL), despite potent in vitro activity4,5.Recently, it was found that some of dietary flavonoids and vitamin C have antagonistic interaction with bortezomib which affects the anticancer property of this drug6-9.According to that 77% of patients use vitamins or herbs concurrently with conventional anticancer treatment10, here we review current knowledge concerning how dietary intake could counteract chemotherapy with bortezomib.
Chemical structure and anti-cancer properties of bortezomib
Bortezomib is a modi fied dipeptidyl boronic acid.The product is provided as a mannitol boronic ester which, in reconstituted form,consists of the mannitol ester in equilibrium with its hydrolysis product, the monomeric boronic acid (Figure 1).One of the first proteasome inhibitors synthesized was MG-132, a peptide aldehyde based on calpain inhibitor I11.However, MG-132 was found to be nonselective because it inhibits other enzymes.Using a boronic acid instead of an aldehyde circumvents theses hortcomings and provides a measure of selective proteasome inhibition relative to many other serine proteases2,12.

Figure 1 Basic chemical structures of bortezomib (A) and MG-132 or MG-262 (B).
Previous studies suggested that proteasome inhibition by bortezomib kills multiple myeloma cells via blocking inducible I-κB degradation and consequently NF-κB activation implicated as one of the mechanisms of tumor cell resistance to apoptosis1,13,14.It induces cell cycle arrest and apoptosis in small cancer cells by preventing degradation of p21/waf1, a cyclindependent kinase inhibitor 1, and p5315.In vitro experiment demonstrated that bortezomib also prevents degradation of Bax,a short-lived pro-apoptotic protein, in CLL and diffuse large B-cell lymphoma (DLBCL) cells16.Malignant cells can resist by failing to accumulate pro-apoptotic proteins after bortezomib treatment, and/or increase the levels of anti-apoptotic proteins,inducing autophagy to clear up damaged proteins17.
Chemical structure and classi fication of flavonoids
Flavonoids are biologically active polyphenolic compounds with various health benefits, ubiquitously found in fruits,vegetables, tea, and wine.Flavonoids are benzo-γ-pyrone derivatives consisting of phenolic and pyrane rings (Figure 2)and are classified according to substitutions, including flavonols (e.g., quercetin, kaempferol), flavones (e.g., apigenin,luteolin), flavanones (e.g., hesperidin, naringenin), flavan-3-ols (e.g., catechin, theaflavin, and gallic esters of catechin and theaflavins), anthocyanidins (e.g., pelargonidin, cyanidin),and isoflavones (e.g., genistein, daidzein)18,19.They also have different distribution of hydroxyl groups (-OH) in their B ring.For example, quercetin is a catechol with 2 hydroxyl groups(-OH) on neighbouring carbon atoms of their B rings; and myricetin, a pyrogallol, has 3-OH groups, whereas both apigenin and kaempferol have only one isolated -OH group on the B ring (Figure 3A).There are many flavonoids containing either catechol or pyrogallol structures in human food sources, such as green vegetables, green tea and fruits (Table 1).Interestingly,vitamin C (L-ascorbic acid), an analogue of catechol, contains a vicinal diol group or two neighbouring hydroxyl groups(Figure 3B).The hydroxyl configuration on the B-ring of flavonoids and in the vicinal diol group of vitamin C is the most significant determinant of scavenging of reactive oxidative species (ROS)18,20,21.Hydroxyl groups on the B-ring donate hydrogen and an electron to hydroxyl, peroxyl, and peroxynitrite radicals, stabilizing them and giving rise to a relatively stable flavonoid radical18.However, the complex formation of the vicinal diol in catechol and vitamin C and its simple derivatives with boron acid in aqueous solution has been well characterized chemically for several decades8,20.
Figure 2 Basic chemical structure of flavonoids.
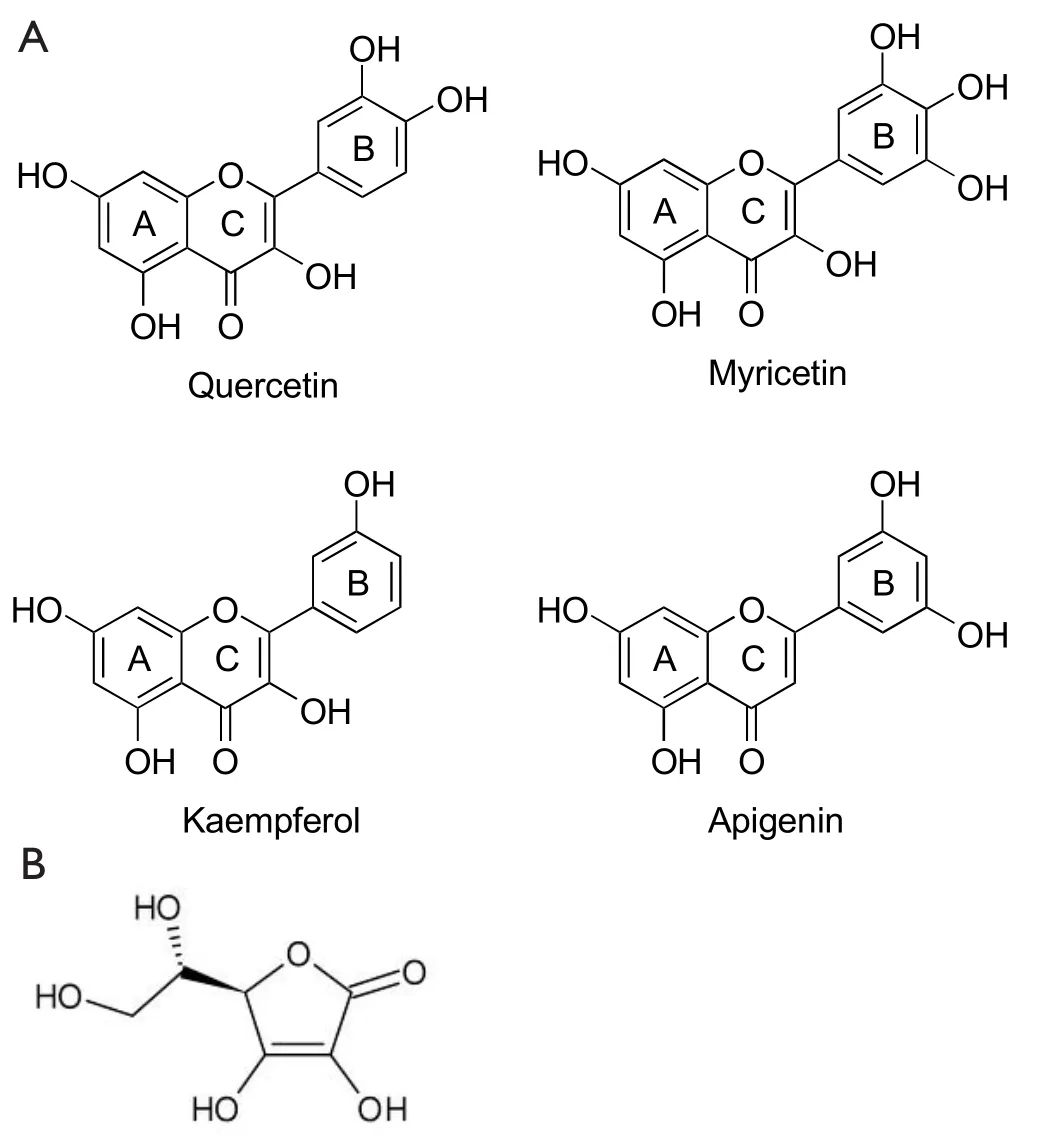
Figure 3 A.Chemical structure of quercetin, myricetin, kaempferol,and apigenin.The B-rings of quercetin and myricetin are catechol and pyrogallo respectively.B.Chemical structure of vitamin C or ascorbic acid.
Flavonoids quercetin and myricetin diminish the anti-cancer effects of bortezomib
Bortezomib does not kill leukemic cells in vivo, despite its potent cytotoxicity in vitro4.To mimic in vivo environment, Liu et al.8found that the efficacy of bortezomib dramatically compromised when CLL cells were cultured in 50% fresh human plasma compared to culturing in 10% fetal calf serum, suspecting that unknown antagonistic compounds against bortezomib exist in the blood.Quercetin is one of the abundant flavonol-type flavonoids, commonly found in green leaves of vegetables and fruits.The average daily intake of flavonoids (quercetin, myricetin,kaempferol) and two other flavone-type flavonoids (apigenin and luteolin), was estimated to be 23 mg/day, with quercetin(mean intake, 16 mg/day) as the most consumed of these five flavonoids22.Quercetin is rich in the plasma and is extensively plasma-bound, almost exclusively to human serum albumin23.The plasma concentration of quercetin is tightly associated with its dietary intake24.Quercetin has many functional similarities to bortezomib in the treatment of cancer cells, such as inhibiting proteasome and NF-κB, inducing apoptosis and cell cycle arrest(Table 2).However, when treating CLL cells with quercetin and bortezomib simultaneously, the apoptosis-inducing effects of both compounds were vanished completely8.It was thought that quercetin-mediated antagonism on bortezomib was due to inhibition of ROS generation.N-acetylcysteine, a ROS scavenger,failed to inhibit bortezomib-induced cells death, instead enhanced its cytotoxicity.A similar result was produced by another group7.The boronic acid group, -B(OH)2, which is present in bortezomib,can be expected to form cyclic boronate esters with the catechols and pyrogallols groups (Figure 4A), but not with flavonoids such as apigenin and kaempferol in which pairs of adjacent hydroxyl groups are absent (Figure 3A).Using Roman spectrophotometry,Liu et al., confirmed the directly chemical binding between quercetin and bortezomib (Figure 4B).
As expected, myricetin showed a similar blocking effect on bortezomib-induced apoptosis as quercetin did but neither apigenin nor kaempferol interfered with bortezomib.The inhibitory effects of plasma on bortezomib cannot be attributed solely to quercetin as its reported peak serum concentration after a supplemental diet is too low32.However, there are many dietary flavonoids that have similar structures with quercetin or myricetin (Table 1), so the intake of dietary flavonoids mayreduce the killing activity of bortezomib on circulating leukemic cells by the formation of a boronate complex.
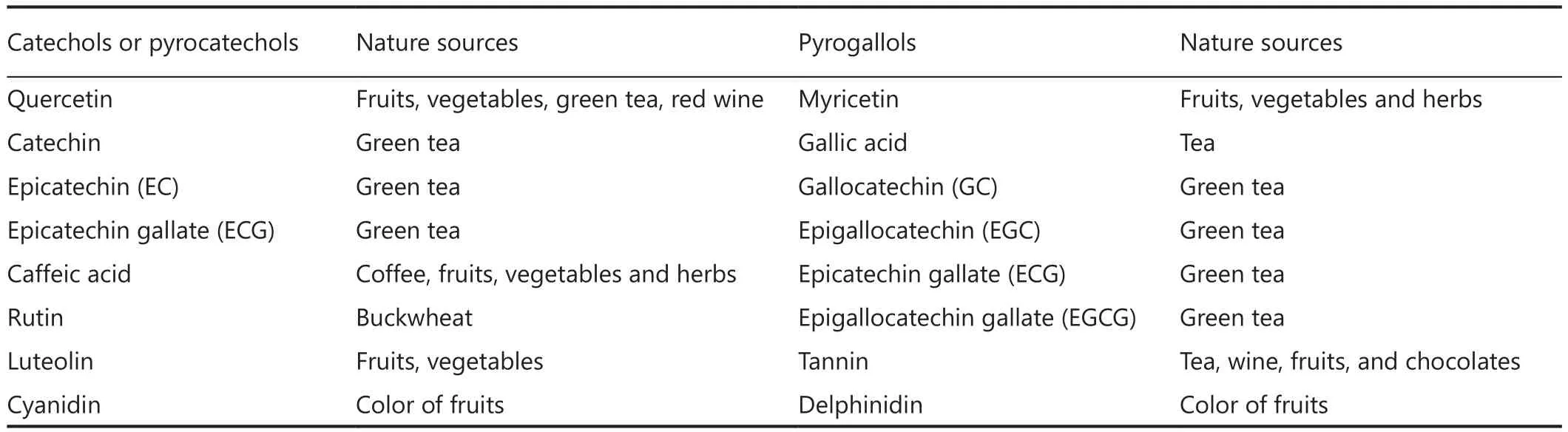
Table 1 Dietary flavonoids with catechol and pyrogallol structures
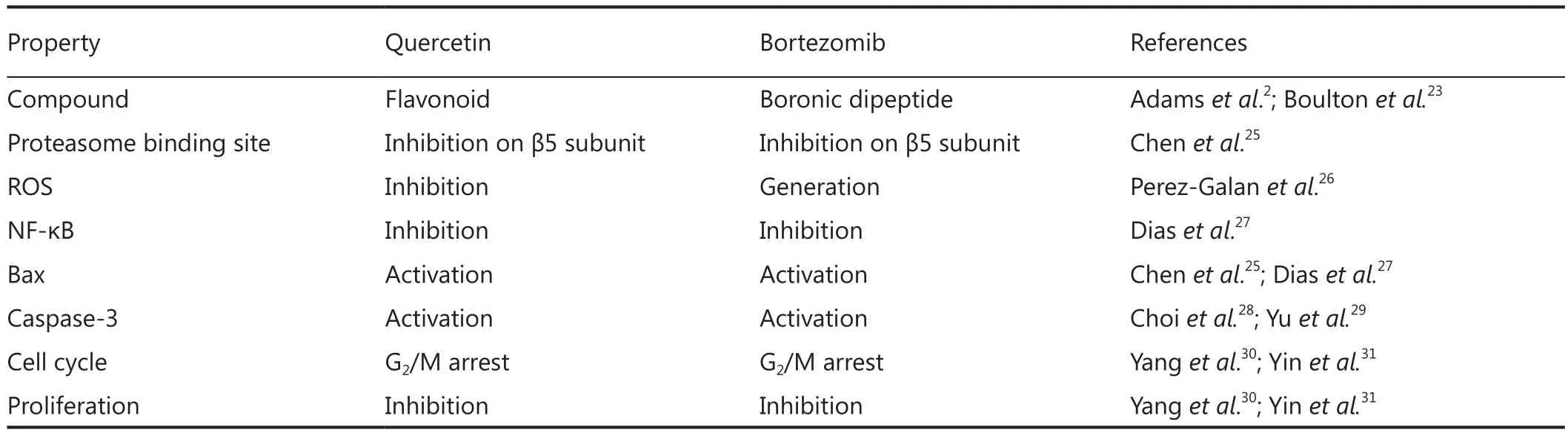
Table 2 Comparison of apoptosis-inducing effects of quercetin and bortezomib

Figure 4 A.Complex formation between catechol derivatives and boronic acid to form boronate ester.B.Detection of chemical reactive between bortezomib and quercetin by Raman Spectrophotometry.
This study con firmed that the apoptotic effect of MG-262, a boron acid-containing proteasome inhibitor (Figure 1B), can also be diminished by quercetin.By contrast, the cytotoxic effect of MG-132 which does not contain boron acid was not affected by quercetin.Importantly, to neutralize flavonoids, inorganic boric acid was added into autologous plasma before treatment with bortezomib.In a non-cytotoxic dose range, boric acid restored apoptosis-inducing activity of bortezomib in a dosedependent manner8.
Flavonoids in green tea block anti-cancer effect of bortezomib and other boronic acid-based proteasome inhibitor
The health benefits and cancer prevention/anti-cancer effects of green tea components have drawn great attention for over two decades due to their anti-oxidant property33-36.The antioxidant compounds in green tea, including Gallocatechin(GC), Epigallocatechin (EGC), Epicatechin gallate (ECG),and Epigallocatechin gallate (EGCG), are pyrogallo-based compounds and EGC contains extra catechol ring (Figure 5).EGCG, the most bioactive green tea polyphenol has been proposed as a multi-functional chemoprevention and anti-cancer agent33,37-40.
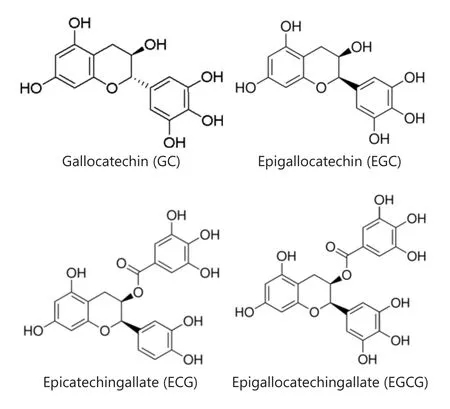
Figure 5 Chemical structures of green tea compounds.
Golden et al.6initially found that 10 µM of EGCG completely blocked 10 nM bortezomib-induced apoptosis in primary and multiple myeloma cell lines, and also in glioblastoma cell lines.EGCG from drug store, called TEA-VIGO, produced similar results on blocking bortezomib as the chemical from Sigma.Other green tea polyphenol components, including EGC, ECG,and EC, all showed blocking effects on bortezomib but required relatively higher concentrations.Complete green tea extract(GTE) also blocked the killing by bortezomib regardless the cytotoxic effect of GTE alone at higher concentration.Similar to the study Liu et al.8, Golden et al., also tested the antagonistic effect of EGCG on other proteasome inhibitors which either contain boronic acid such as Nelfinavir, PS-1 and MG-132 or without it, such as MG-262 and PX-1X.They found that the protective feature of EGCG is not a general effect toward all proteasome inhibitors, but rather displays selectivity toward those compounds harboring a boronic acid moiety.For in vivo study, multiple myeloma cells were implanted subcutaneously into nude mice and, after sizable tumors had formed, the animals received treatment with both bortezomib and/or EGCG.In tumors from animals treated with EGCG (25 to 50 mg/kg) or EGCG plus bortezomib, there is no increase of apoptotic cells compared with tumors from untreated controls.Finally, using1H NMR and1C NMR techniques they confirmed that direct interaction between EGCG and bortezomib lead to formation of a covalent cyclic boronate between these two compounds6.
Vitamin C inhibits anti-cancer effect of bortezomib
Vitamin C or L-ascorbic acid is the most common healthy food supplement for both normal human being and cancer patients.Structurally, it is not a flavonoid, or polyphenol but it contains a vicinal diol (Figure 3B) and has a strong antioxidant activity.Currently, the effect of vitamin C in cancer prevention and influence on chemotherapeutic drugs remain controversial41-43.
Perrone et al.9observed that vitamin C blocks bortezomibmediated growth inhibition, accumulation of ubiquitinated proteins and inhibition of proteasome activity in multiple myeloma cell lines.This antagonistic effect of vitamin C on bortezomib is limited to peptide boronic class proteasome inhibitors.This group conducted in vivo study in xenograft mouse model of human multiple myeloma by treating mice with vitamin C or bortezomib alone or in combination.Bortezomib alone significantly inhibited tumor growth while vitamin C alone showed no effect.Importantly, vitamin C (40 mg/kg/day)completely blocked bortezomib-mediated anti-cancer effect.
Effects of non-vicinal diol containing naturally occurring compounds on bortezomib
Previous study by Liu et al.8, demonstrated that non-vicinal diol containing flavonoids such as kaempferol or apignin don’t have antagonistic effect on bortezomib.Flavopiridol is a synthetic flavonoid based on an extract from an Indian plant for the potential treatment of cancer.It was found that flavopiridol(Alvacidib) has synergistic effect on bortezomib-induced killing in chronic myeloid leukemia44.A phase I study showed that bortezomib/ flavopiridol regimen appears active in patients with relapsed and/or refractory multiple myeloma or non-Hodgkin’s lymphoma45.Curcumin is a natural occurring flavonoid extracted from Indian spice turmeric.It was found that curcumin and its analogs enhanced bortezomib-induced apoptosis in multiple myeloma cells46,47.Resveratrol, a nature phenol, is produced by Japanese knotweed, red grapes, berries, and peanuts and is also found at high concentration in red wine.It was reported that resveratrol mediates apoptosis in multiple myeloma cells when used alone or in combination with paclitaxel48or bortezomib49.However, a phase II study of SRT501 (a micronized oral formulation of resveratrol) with bortezomib found that SRT501 causes renal failure and minimal efficacy in patients with relapsed/refractory multiple myeloma when used alone or in combination with bortezomib50.
Preclinical in vivo studies and controversies
A preclinical study on the antagonistic effect of EGCG or vitamin C in the anti-tumor activity of bortezomib was conducted by Millennium Pharmaceuticals using CWR22 human prostate xenograft tumors51.Experiment using multiple myeloma cell line RPMI8226 showed that the concentration of EGCG required for partially inhibiting bortezomib was≥11 µM, higher than previously reported, e.g., 10 µM for complete inhibition6.The plasma concentrations of EGCG were monitored after intravenous (IV) administration of EGCG.Single-agent bortezomib 0.8 mg/kg IV demonstrated a tumor growth inhibition (TGI) of 53.9%-58.9% versus the control group in CWR22 xenograft-bearing mice.EGCG 50 mg/kg IV administered two min prior to bortezomib 0.8 mg/kg IV resulted in no antitumor activity, demonstrating antagonism between EGCG and bortezomib when EGCG levels were>200 µM at the time of bortezomib dosing.Pharmacodynamic studies were conducted to determine whether EGCG showed a concentration-dependent ability to antagonize bortezomibinduced proteasome inhibition in blood or tumor tissue.Four hours after IV administration of bortezomib 0.8 mg/kg,mean 20S proteasome inhibition was 44% in blood and 52%in tumor.This proteasome inhibition was blunted by the combination of EGCG 50 mg/kg IV followed 2 min later by bortezomib 0.8 mg/kg IV, which resulted in only 25% and 33%proteasome inhibition in blood and tumor, respectively, at 4 h post-administration.Golden et al., found that 25 or 50 mg/mL EGCG blocked the apoptosis-inducing effect of 0.5 mg/mL of bortezomib in nude mice.These results indicate partial or complete antagonism of proteasome inhibition by IV dosing of EGCG, which is further re flected in the analysis of downstream pharmacodynamic markers and ultimately in the antagonism of antitumor activity in this combination regimen.
This group also determined the antagonistic effect of vitamin C on bortezomib.Ascorbic acid 40 or 500 mg/kg alone did not exhibit any antitumor activity, while bortezomib 0.8 mg/kg IV alone had significant antitumor activity compared with controls.Surprisingly, they found that ascorbic acid 40 or 500 mg/kg in combination with bortezomib also exhibited significant antitumor activity compared with controls.No antagonism was seen between ascorbic acid and bortezomib in any of the combination groups.This is contradicted with previous study by Perrone et al.9, which demonstrated that 40 mg/kg ascorbic acid abolished 0.1 mg/kg bortezomib-induced tumor growth inhibition in human multiple myeloma xenograft-bearing mice.It remains unexplained why different xenograft animal models and different ratios of drugs produce different results.
This study51concluded that plasma concentrations of EGCG and ascorbic acid reported in human subjects taking EGCG or vitamin C supplements show no antagonism to the antitumor activity of bortezomib in human prostate tumor fragment xenograft-bearing mice, and therefore there appears no need for patients receiving bortezomib therapy to avoid normal dietary consumption of green tea, vitamin C-containing foods, or EGCG or vitamin C dietary supplements.
Unanswered questions and perspectives
Although the direct in vitro interaction between vicinal diol inflavonoids/vitamin C and boronic acid was detected by several groups6-8, the differential interaction potential in plasma and solid tumor environment was not determined.It is still unclear why bortezomib can kill malignant cells in the tissue and bone marrow but not in the blood.If the anti-cancer property of bortezomib is affected by flavonoids in the blood, how it can reach to the tumor or bone marrow sites unaffected?
Some natural products or antioxidants such as luteolin, ellagic acid, flavonoids, protocatechuic acid, rosmarinic acid, phenethyl caffeate and catechin from vegetables, fruits or herbs have one or more vicinal diol groups.Thus these agents may have the potential to chemically interact with bortezomib and antagonize its activity20.Nonetheless, these studies serve as an always timely reminder for healthcare providers of the importance of eliciting a complete history from patients and their families, including concomitant medications and over-the-counter supplements52.It is reasonable to suggest to patients that there are potentially negative interactions between proven anticancer therapies and ‘complementary’ therapies.Until we, as researchers and clinicians, have a clear understanding of the potential interactions or lack thereof, we should caution our patients to limit their use to maximize their bene fit from treatment20.
There are several strategies which may allow further development of proteasome inhibitors for the treatment of leukemia8,53.First, decreasing plasma flavonoid concentrations by dietary manipulation may, if achievable, be of value in enhancing in vivo toxicity of bortezomib.Second, the observation that boric acid can compete with the reaction between quercetin and bortezomib raises the possibility that the blocking effect of flavonoids may be neutralized prior to bortezomib treatment.A third option would be to explore the possible therapeutic use of proteasome inhibitors which lack a boronate moiety.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ,Adams J, et al.The proteasome inhibitor PS-341 inhibits growth,induces apoptosis, and overcomes drug resistance in human multiple myeloma cells.Cancer Res 2001;61:3071-3076.
2.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A,Lazarus DD, et al.Proteasome inhibitors: a novel class of potent and effective antitumor agents.Cancer Res 1999;59:2615-2622.
3.Zhang J, Shen L, Wang J, Luo P, Hu Y.Design, Synthesis and Biological Evaluation of Novel Non-Peptide Boronic Acid Derivatives as Proteasome Inhibitors.Med Chem 2013.[Epub ahead of print].
4.Faderl S, Rai K, Gribben J, Byrd JC, Flinn IW, O’Brien S, et al.Phase II study of single-agent bortezomib for the treatment of patients with fludarabine-refractory B-cell chronic lymphocytic leukemia.Cancer 2006;107:916-924.
5.Ruiz S, Krupnik Y, Keating M, Chandra J, Palladino M,McConkey D.The proteasome inhibitor NPI-0052 is a more effective inducer of apoptosis than bortezomib in lymphocytes from patients with chronic lymphocytic leukemia.Mol CancerTher 2006;5:1836-1843.
6.Golden EB, Lam PY, Kardosh A, Gaffney KJ, Cadenas E, Louie SG, et al.Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors.Blood 2009;113:5927-5937.
7.Kim TY, Park J, Oh B, Min HJ, Jeong TS, Lee JH, et al.Natural polyphenols antagonize the antimyeloma activity of proteasome inhibitor bortezomib by direct chemical interaction.Br J Haematol 2009;146:270-281.
8.Liu FT, Agrawal SG, Movasaghi Z, Wyatt PB, Rehman IU, Gribben JG, et al.Dietary flavonoids inhibit the anticancer effects of the proteasome inhibitor bortezomib.Blood 2008;112:3835-3846.
9.Perrone G, Hideshima T, Ikeda H, Okawa Y, Calabrese E, Gorgun G, et al.Ascorbic acid inhibits antitumor activity of bortezomib in vivo.Leukemia 2009;23:1679-1686.
10.Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE.Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology.J Clin Oncol 2000;18:2505-2514.
11.Tsubuki S, Kawasaki H, Saito Y, Miyashita N, Inomata M,Kawashima S.Purification and characterization of a Z-Leu-Leu-Leu-MCA degrading protease expected to regulate neurite formation: a novel catalytic activity in proteasome.Biochem Biophys Res Commun 1993;196:1195-1201.
12.Adams J.The development of proteasome inhibitors as anticancer drugs.Cancer Cell 2004;5:417-421.
13.Chauhan D, Singh A, Brahmandam M, Podar K, Hideshima T, Richardson P, et al.Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma.Blood 2008;111:1654-1664.
14.Ma MH, Yang HH, Parker K, Manyak S, Friedman JM, Altamirano C, et al.The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents.Clin Cancer Res 2003;9:1136-1144.
15.Yang Y, Ikezoe T, Saito T, Kobayashi M, Koeラer HP, Taguchi H.Proteasome inhibitor PS-341 induces growth arrest and apoptosis of non-small cell lung cancer cells via the JNK/c-Jun/AP-1 signaling.Cancer Sci 2004;95:176-180.
16.Liu FT, Agrawal SG, Gribben JG, Ye H, Du MQ, Newland AC, et al.Bortezomib blocks Bax degradation in malignant B cells during treatment with TRAIL.Blood 2008;111:2797-2805.
17.Jia L, Gopinathan G, Sukumar JT, Gribben JG.Blocking autophagy prevents bortezomib-induced NF-κB activation by reducing I-κBα degradation in lymphoma cells.PLoS One 2012;7:e32584.
18.Heim KE, Tagliaferro AR, Bobilya DJ.Flavonoid antioxidants:chemistry, metabolism and structure-activity relationships.J Nutr Biochem 2002;13:572-584.
19.Romagnolo DF, Selmin OI.Flavonoids and cancer prevention: a review of the evidence.J Nutr Gerontol Geriatr 2012;31:206-238.
20.Harvey RD, Nettles J, Wang B, Sun SY, Lonial S.Commentary on Perrone et al.: ‘vitamin C: not for breakfast anymore...if you have myeloma’.Leukemia 2009;23:1939-1940.
21.Sekher Pannala A, Chan TS, O’Brien PJ, Rice-Evans CA.Flavonoid B-ring chemistry and antioxidant activity: fast reaction kinetics.Biochem Biophys Res Commun 2001;282:1161-1168.
22.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D.Dietary antioxidant flavonoids and risk of coronary heart disease:the Zutphen Elderly Study.Lancet 1993;342:1007-1011.
23.Boulton DW, Walle UK, Walle T.Extensive binding of the bio flavonoid quercetin to human plasma proteins.J Pharm Pharmacol 1998;50:243-249.
24.Mullen W, Boitier A, Stewart AJ, Crozier A.Flavonoid metabolites in human plasma and urine after the consumption of red onions:analysis by liquid chromatography with photodiode array and full scan tandem mass spectrometric detection.J Chromatogr A 2004;1058:163-168.
25.Chen D, Daniel KG, Chen MS, Kuhn DJ, Landis-Piwowar KR, Dou QP.Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells.Biochem Pharmacol 2005;69:1421-1432.
26.Pérez-Galán P, Roué G, Villamor N, Montserrat E, Campo E,Colomer D.The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status.Blood 2006;107:257-264.
27.Dias AS, Porawski M, Alonso M, Marroni N, Collado PS,González-Gallego J.Quercetin decreases oxidative stress,NF-kappaB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats.J Nutr 2005;135:2299-2304.
28.Choi YJ, Jeong YJ, Lee YJ, Kwon HM, Kang YH.(-)Epigallocatechin gallate and quercetin enhance survival signaling in response to oxidant-induced human endothelial apoptosis.J Nutr 2005;135:707-713.
29.Yu J, Tiwari S, Steiner P, Zhang L.Differential apoptotic response to the proteasome inhibitor Bortezomib [VELCADE, PS-341]in Bax-de ficient and p21-de ficient colon cancer cells.Cancer BiolTher 2003;2:694-699.
30.Yang JH, Hsia TC, Kuo HM, Chao PD, Chou CC, Wei YH, et al.Inhibition of lung cancer cell growth by quercetin glucuronides via G2/M arrest and induction of apoptosis.Drug Metab Dispos 2006;34:296-304.
31.Yin D, Zhou H, Kumagai T, Liu G, Ong JM, Black KL, et al.Proteasome inhibitor PS-341 causes cell growth arrest and apoptosis in human glioblastoma multiforme (GBM).Oncogene 2005;24:344-354.
32.Hollman PC, van Trijp JM, Mengelers MJ, de Vries JH, Katan MB.Bioavailability of the dietary antioxidant flavonol quercetin in man.Cancer Lett 1997;114:139-140.
33.Agarwal R, Katiyar SK, Zaidi SI, Mukhtar H.Inhibition of skin tumor promoter-caused induction of epidermal ornithine decarboxylase in SENCAR mice by polyphenolic fraction isolated from green tea and its individual epicatechin derivatives.Cancer Res 1992;52:3582-3588.
34.Fujiki H, Yoshizawa S, Horiuchi T, Suganuma M, Yatsunami J,Nishiwaki S, et al.Anticarcinogenic effects of (-)-epigallocatechin gallate.Prev Med 1992;21:503-509.
35.Katiyar SK, Agarwal R, Mukhtar H.Green tea in chemoprevention of cancer.Compr Ther 1992;18:3-8.
36.Wang ZY, Huang MT, Ferraro T, Wong CQ, Lou YR, Reuhl K, et al.Inhibitory effect of green tea in the drinking water on tumorigenesis by ultraviolet light and 12-O-tetradecanoylphorbol-13-acetate in the skin of SKH-1 mice.Cancer Res 1992;52:1162-1170.
37.Bhimani RS, Troll W, Grunberger D, Frenkel K.Inhibition of oxidative stress in HeLa cells by chemopreventive agents.Cancer Res 1993;53:4528-4533.
38.Mak JC.Potential role of green tea catechins in various disease therapies: progress and promise.Clin Exp Pharmacol Physiol 2012;39:265-273.
39.Singh BN, Shankar S, Srivastava RK.Green tea catechin,epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications.Biochem Pharmacol 2011;82:1807-1821.
40.Smith DM, Daniel KG, Wang Z, Guida WC, Chan TH, Dou QP.Docking studies and model development of tea polyphenol proteasome inhibitors: applications to rational drug design.Proteins 2004;54:58-70.
41.Ettari R, Bonaccorso C, Micale N, Heindl C, Schirmeister T,Calabrò ML, et al.Development of novel peptidomimetics containing a vinyl sulfone moiety as proteasome inhibitors.Chem Med Chem 2011;6:1228-1237.
42.Lawrence HR, Kazi A, Luo Y, Kendig R, Ge Y, Jain S, et al.Synthesis and biological evaluation of naphthoquinone analogs as a novel class of proteasome inhibitors.Bioorg Med Chem 2010;18:5576-5592.
43.Marzaro G, Gandin V, Marzano C, Guiotto A, Chilin A.Psoralenquinones as a novel class of proteasome inhibitors: design,synthesis and biological evaluation.Chem Med Chem 2011;6:996-1000.
44.Harding CV, France J, Song R, Farah JM, Chatterjee S, Iqbal M, et al.Novel dipeptide aldehydes are proteasome inhibitors and block the MHC-I antigen-processing pathway.J Immunol 1995;155:1767-1775.
45.Gantt SM, Myung JM, Briones MR, Li WD, Corey EJ, Omura S, et al.Proteasome inhibitors block development of Plasmodium spp.Antimicrob Agents Chemother 1998;42:2731-2738.
46.Fang H, Hu Z, Zhou G.Establishment of a GFP-based cellular model for screening novel proteasome inhibitors.Sheng Wu Gong Cheng Xue Bao 2009;25:452-456.
47.Mujtaba T, Kanwar J, Wan SB, Chan TH, Dou QP.Sensitizing human multiple myeloma cells to the proteasome inhibitor bortezomib by novel curcumin analogs.Int J Mol Med 2012;29:102-106.
48.Roginsky AB, Ujiki MB, Ding XZ, Adrian TE.On the potential use of flavonoids in the treatment and prevention of pancreatic cancer.In Vivo 2005;19:61-67.
49.Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y,Gaur U, et al.Resveratrol inhibits proliferation, induces apoptosis,and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells.Blood 2007;109:2293-2302.
50.Popat R, Plesner T, Davies F, Cook G, Cook M, Elliott P, et al.A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma.Br J Haematol 2013;160:714-717.
51.Bannerman B, Xu L, Jones M, Tsu C, Yu J, Hales P, et al.Preclinical evaluation of the antitumor activity of bortezomib in combination with vitamin C or with epigallocatechin gallate, a component of green tea.Cancer Chemother Pharmacol 2011;68:1145-1154.
52.Shah JJ, Kuhn DJ, Orlowski RZ.Bortezomib and EGCG: no green tea for you? Blood 2009;113:5695-5696.
53.Wickremasinghe RG.Why is CLL refractory to bortezomib? Blood 2008;112:3540-3541.
杂志排行
Cancer Biology & Medicine的其它文章
- Asian trends in primary androgen depletion therapy on prostate cancer
- Therapeutic resistance in cancer: microRNA regulation of EGFR signaling networks
- Effects of HLEC on the secreted proteins of epithelial ovarian cancer cells prone to metastasize to lymph nodes
- Analysis of 30 patients with persistent or recurrent squamous cell carcinoma of the cervix within one year after concurrent chemoradiotherapy
- Rare myeloid sarcoma/acute myeloid leukemia with adrenal mass after allogeneic mobilization peripheral blood stem cell transplantation
- Instructions for Authors
