氨三乙酸、缬氨酸、亮氨酸的三元过渡金属配合物:合成和生物学应用
2013-09-15MostafaKhalilEglalSouayaEmanIsmailEmanRabie
Mostafa M H Khalil Eglal R Souaya Eman H Ismail*,,2 Eman Rabie
(1Chemistry Department,Faculty of Science,Ain Shams University,11566,Abbassia,Cairo,Egypt.(埃及)
(2Chemistry Department,Faculty of Science,Taibah University,Al-Madina Al-Munawarah,B.O:344.,Kingdom of Saudi Arabia)(沙特阿拉伯王国)
0 Introduction
Ternary complexes of metal(Ⅱ)containing nitrogen and oxygen-donor ligands have received considerable attention newly because they could achieve exceptionally high stability[1-3].In the other hand,ternary metal complexeshavebeen recently studied duetotheir power as metal systems for metal-protein complexes such as metalloenzymes.They have been received particular attention and have been used in cartographic protein surfaces[4]for biological redox centers[5]and in protein pick up for both purification[6]and studying[7-8].
As a strong example for ternary metal complexes,the high binding affinity of Ni2+to multiple histidine(His)sitesisemployed to label His-tagged PsbH protein(protein belongs to a group of small protein subunits of photosystem II(PSII)complex)[9].
On the other hand,nickel plays a key role in methane formation where nitrilotriacetic acid(NTA)improves the methane production because NTA favors the dissolution of nickel from their carbonates and sulphides.Thus a direct uptake of the complex NTA-Ni might have occurred and methane production is significantly improved[10].
Also Ternary complexes of cobalt(Ⅱ) with nitrilotriacetic acid as a primary ligand and some selected mono-and dicarboxylic acids as secondary ligands had been synthesized.These complexes were formed in a stepwise mechanism[11].
NTA has numerous commercial applications as a metal ion chelator,including principally its use in cleaning products,industrial water treatment,textile preparation,metal finishing and in agricultural herbicide formulations and micronutrient solutions.It has also been used in the pulp and paper industry.In addition,somemetal NTA complexeshave been used in the rubber processing,photographic products,the electrochemical industry,the tanning of leather,and in cosmetics[12-13].Moreover,it has been evaluated as a soil additive in the phytoremediation of heavy metal contaminated soil[14]..The chelation of the metals with nitrilotriacetic acid is to mo metals for more rapid uptake by plants.Nitrilotriacetic acid has been suggested for use as a therapeutic chelating agent for the treatment of manganese poisoning[15]and iron overloading[16].
For the great biological applications,much of the studies on the coordination of nitrilotriacetic acid with transition metal to formbinary or ternary complexeshas been done in neutral or slightly basic media,where such ligands are in the ionized form(NTA3-)[17-20].
The amino ternary metal complexes have attracted much attention because they are useful as antibacterial agents against Staphylococcus aureus,Escherichia coli and Candida albicans.Also they are used as antitumoral drugs against sarcoma and leukaemia;nutritive supplies for human and animals[21-22].Leucine is an essential branched-chain amino acid used as a source for the synthesis of blood sugar in the liver during starvation,stress and infection to aid in healing[23].
Synthesis,structure,DNA binding and oxidative cleavage activity of ternary (L-leucine/isoleucine)copper(Ⅱ)complexes of heterocyclic bases have been studied[24].Also synthesis of a new copper(Ⅱ)complex with L-valine Schiff base and 1,10-phenanthroline[Cu(sal-L-val)phen](sal-L-val=a Schiff base derived from salicylaldehyde and L-valine,phen=1,10 phenanthroline)was studied[25].Two new complexes of cobalt(Ⅱ)and copper(Ⅱ) with valine-derived Schiff bases were prepared.Biological studies for all these complexes were carried out in vitro for antimicrobial activity against Gram-positive,Gram-negative bacteria and human pathogenic fungi to give great results against pathogenic microorganisms[25-26].
In the previous study,ternary complex of Co(Ⅱ),Ni(Ⅱ),Cu(Ⅱ),and Zn(Ⅱ) with nitrilotriacetic acid as a primary ligand and alanine or phenylalanine as secondary ligands in slightly acidic medium were prepared and their molecular structures were found to be[M(HNTA)(alaH)(H2O)2][27].
In the present paper, synthesis and characterization of new Ni(Ⅱ),Cu(Ⅱ)and Zn(Ⅱ)complexes using NTA as a primary ligand in the presence of valine or leucine as secondary ligand in slightly acidic medium are investigated.The biological studies of the complexesas antibacterial activity against Escherichia coli and Staphylococcus aureus bacteria and antifungal activity toward Aspergillus flavus and Candida albicansfungiare done.
1 Experimental
1.1 Materials and reagents
All chemicals used in this study were reagent grade and used without further purification.H3NTA,valine,and leucine were Sigma-Aldrich products CuCO3·Cu(OH)2·H2O,NiCO3·2Ni(OH)2·4H2O,CoCO3·3Co(OH)2,ZnCO3·2Zn(OH)2·H2Ofrom BDH chemicals were used.
1.2 Synthesisof metal complexes
The calculated amounts of metal carbonate,H3NTA,and valine or leucine to give the 1 ∶1 ∶1 molar ratio were mixed in 100 mL of distilled water,and the mixture was heated nearly to boiling.After the completion of the reaction absolute ethanol was added until dense precipitate was appeared.Then the precipitate was obtained by filtration,washing with alcohol and drying in an oven at 100℃and then placing in a desiccator over night.This procedure was followed for thepreparation of all the1∶1∶1 complexes.
1.3 Biological activities
The antimicrobial activities were carried out by Kirby-Baeur technique.One Aspergillus species(A.flavus),one Candida species (C.albicans)and two Bacteria (Escherichia coli,Staphyllococcus aureus)were used for this study.All isolates were from Microanalytical Center,Faculty of Science,Cairo University.
The agar used was Meuller-Hinton agar rigorously tested for composition and pH value.Nutrient agar was melted at 45℃and inoculated by the microbial suspension (1 mL/100 mL).100 μL of microbial suspension wasspread ontoagar platescorrespondingto the broth in which they were maintained.
Plates were inoculated with filamentous fungi as Aspergillusflavus at 25℃for 48 h;Gram (+)bacteria as Staphylococcus aureus,and Gram (-)bacteria as Escherichia coli were incubated at 35~37℃ for 48 h and yeast as Candida albicans was incubated at 30℃for 48 h.
The standard discs of Tetracycline(antibacterial agent),Amphotericin B (antifungal agent)were served as positive controls for antimicrobial activity but filter discs impregnated with 10μL of solvent(distilled water,chloroform,DMSO)were used as a negative control.
The filter paper disc impregnated with a tested chemical was placed on the agar.The chemicals would diffuse from the disc into the agar.This diffusion will place the chemicals in the agar only around the disc.Thezoneof inhibition diameterswasmeasured.
1.4 Instrumentation
All measurements were carried out at the microanalytical laboratories of Cairo University,Ain Shams University and the National Research Center,Cairo.C,H and N were determined by Vario El Elementar.Ni,Cu and Zn percentages were determined by atomic absorption spectrometry (AAS),using a Perkin-Elmer AAS 3100.IR spectra of the solid complexes were recorded on a Jasco FTIR-300 E Fourier Transform Infrared Spectrometer,using KBr disks in the range of 400~4 000 cm-1and CsI in the range of 200~630 cm-1.Thermogravimetric analysiswas carried out using a Perkin-Elmer 7 series thermal analyzer.The measurements were carried out under nitrogen atmosphere at a heating rate of 10 ℃·min-1.Magnetic susceptibilities of the paramagnetic metal complexes were measured by using a magnetic susceptibility balance Johnson Mtthy,Alfa products;model No MKIat roomtemperature.The electronic UVVis spectra were recorded at room temperature on a Jasco model V-550 UV/Vis spectrophotometer.Mass spectra were recorded at 350℃and 70 eV on a GC/MS Finnigan Mat SSQ 7000 apparatu
2 Resultsand discussion
2.1 Elemental analysisand physical properties
Elemental analysis, physical and chemical propertiesof thesix complexesare given in Table 1.All the prepared complexes have some common features such as thermal decomposition before melting and effervescence as evolution of carbon dioxide on thereaction with sodium bicarbonate.The molecular masses of the six complexes suggest the presence of three water molecules(Table 1).Comparing the 10 Dq values of the complexes of this study with the literature values of Ni,and Cu octahedral complexes,respectively,for the ternary complexes of this study and two water molecules are coordinated to the metal.The pH values of the solution at which all the complexes under study are crystallized are ranged from 2.8 to 4.0.p K a value of leucine is 2.36(carboxyl),9.60 (amino)[28]and that of valine is 2.32(carboxyl),9.62 (amino)[28].In this pH value range,HNTA2-and valine or leucine in their zwitter form predominate.At this pH value,HNTA2-has three coordination sites (N and two COO-)while valine or leucine coordinate in slightly acidic medium via its carboxylic oxygen after being converted into the zwitter ion form (H3N+CH2COO-).This means that under the reaction condition used in this study,the second amino acids coordinated as monodentate ligand.Accordingly,the metal coordinates to three sites from HNTA2-,one from the second amino acids and two water molecules could be coordinated to form an octahedral structure.Fig.1 shows the suggested structure of the 1 ∶1 ∶1 complex.This suggested structure supposes that there are two ionisable protons;one due to uncoordinated COOH group and the other one from the protonated uncoordinated NH2group of amino acids used.In the following sections IR spectra,thermal analysis and mass spectra will be discussed to support the above conclusion about octahedral structure of our complexes.

Table 1 Elemental analysis,mass spectrometry data,and physical properties of the complexes

Fig.1 Proposed structure of our ternary complexes
2.2 IR spectra
The IR spectra of the H3NTA,valine,leucine,and their metal complexes were carried out in the range of 4 000~400 cm-1and the important bands are listed in Table 2.IR spectra of nickel complexes with H3NTA and valine or leucine are shown in Fig.A in supplementary materials.The IR spectrum of nitrilotriacetic acid(H3NTA)showsbandsat 3 041 and1,733 cm-1which are attributed to νOH with intermolecular hydrogen bonding and undissociated carboxylic groups,respectively.The IR spectra of the ternary complexes with valine or leucine as secondary ligand exhibit bands at 1 727 cm-1and 729 cm-1(Table 2)suggesting the presence of non-coordinated free carboxylic group of H3NTA ligand.On the other hand,the ternary complexes with valine exhibit new strong absorption at 1 727 cm-1and 1 729 cm-1for Ni and Cu complexes,respectively.These shiftscan be assigned to the stretching vibrationν(CO)of the coordinated carboxylate group,COOM[29].Similar data for leucine ternary complexesare observed with appropriate shift of COOM due to complex formation (Table 2).The IR spectra of the free ligands show sharp bands at 1 586 and 1 510 cm-1for valine and at 1 587 and 1 514 cm-1for leucine,which are assigned for asymmetric and symmetric stretching vibrations of the carboxylate moiety,respectively.These two bands of the free ligandsareeither shifted to lower or higher frequencies,indicating that these ligands coordinate to the metal ionsviadeprotonated carboxylategroup[30].On theother hand,the IRspectraof valineand leucineshow medium broad bands at 3 148 and 3 108 cm-1,respectively,which can attribute to NH3+group of the amino acid.The IR spectra then suggest that in ternary complexes HNTA2-is a tridentate ligand (two-COO-and one nitrogen),and that the secondary ligands are monodentate(one-COO-).All the prepared complexes exhibit bands in the range of 3 558~3 406 cm-1signifying that H2Omolecules exist in these complexes.The massspectraof the complexessupport the proposed complexes with three water molecules coordinated to thecentral atomto forman octahedral structure.

Table 2 IR data of our ternary complexes(cm-1)
Further elucidation of the participation of nitrogen and carboxylic groups in HNTA2-and the used amino acids was confirmed by recording the IR spectra of the complexes in the range 50~650 cm-1.The ν(MO)frequencies of NTA3-as ligand are at 362,350 and 355 cm-1for Cu,Niand Zn complexes,respectively[24].Thus one may assign the bands in the complexes in the range of 350~377 cm-1to the M-O vibrational frequencies(Table 2).The M-N band frequencies are found in the range of 462~544 cm-1for Cu Ni,and Zn complexes,respectively(Table 2).
2.3 Magnetic momentsand electronic spectra
The UV-Vis spectra of the metal complexes were recorded in distilled H2O solution (10-2mol·L-1).For ternary Ni(Ⅱ) complexes,the magnetic moments μeffof[Ni(HNTA)(valine)(H2O)2]·1.5H2O and[Ni(HNTA)(leucine)(H2O)2]·H2Ohave values of 4.75 μB(B.M.)and 4.13 μB,respectively,which suggest an octahedral geometry[25].The electronic spectrum of Ni(Ⅱ) complexes displaystwo bandsin the range of 626 nm(15 974 cm-1)and 394 nm(25 380 cm-1)assigned as3A2g→3T1g(F)and3A2g→3T1g(P),respectively.The mixed-ligand complexes of Cu(Ⅱ),with valine and leucine show magnetic moment values, μeff,of 2.61 and 2.36 μBThese values correspond to one unpaired electron and thus offer evidence for mononuclear structures of the complexes.The UV-Vis spectrum of Cu(Ⅱ)complexes consists of a broad band centered at 812 nm(12 315 cm-1)that is assigned to2Eg→2T2gtransition with expected splittingof these states as a result of tetragonal distortion of the octahedral Cu(Ⅱ)ion,d9[27].
2.4 Massspectra
The mass spectra of the six complexes were recorded.All the spectra contain molecular ion peaks and confirm the molecular mass of the complexes.The dataarepresented in(Table1).
2.5 Thermal analysis
Thermogravimetric analysis results are shown in Table 3.And Thermogravimetric analysis curves are given in Fig.B in supplementary materials.Interpretation of the thermal mass losses shows that the complexes thermally decompose in the same patterns exhibited by the parent ligands.Scheme.1 is shown that H3NTAthermally decomposein twooverlappingsteps[31],into glycine and maleic acid.Also,scheme 2 and scheme 3 are shown the supposed thermal decomposition for valine and lucine,respectively.
Both thermal products,on further heating,decompose to give different organic residues overlapping steps.The first mass loss in the complexes is equivalent to the water molecules which supports the suggested structure for the complexes[32]where the decomposing temperatures are very close to the coordinate water temperature decomposition.As the thermal decomposition is made under nitrogen atmosphere and that almost all products are gases,(CO,C2H2and HC≡CH)which interpretsthe liberation of the metal or its carbide formas shown in Table 3.The finalthermal decomposition residue is the mixture of metal or metal carbide with carbon monooxide gas where thermal decomposition is made under nitrogen atmosphere[31,33].

Table 3 Temperature values for the decomposition along with the species lost in each step

Scheme 1 Thermal decomposition of nitrilotriacetic acid[30]

Scheme 2 Thermal decomposition of valine acid[30].

Scheme 3 Thermal decomposition of L-lucine acid[30]
2.6 Biological study
In testing the antimicrobial activity of these compounds,we used more than one test organism to increase the chance of detecting antibiotic principles in the tested materials.The sensitivity of a microorganism to antibiotics and other antimicrobial agents was determined by the assay plates which were incubated at 37℃for 48 h for bacteria,at 25℃for 48 h for Aspergillus flavus and yeast as Candida albicans incubated at 30℃for 48 h.All the Ni and Cu tested compounds show a remarkable biological activity against different types of Gram-positive(G+)bacteria and Gram-negative(G-)bacteria.Only Ni·NTA·valine complex shows a biological activity against Candida albicans that can cause Candidiasis.Candidiasis encompasses infections that range from superficial,such as oral thrush and vaginitis,to systemic and potentially life-threatening diseases.The data are listed in Table 4.The data show that metal complexes under investigation have the capacity of inhibiting the metabolic growth of the investigated bacteria and fungi to different extents.The size of the inhibition zone depends upon the culture medium, incubation conditions,rate of diffusion,and the concentration of the antibacterial agent.The activities of all the tested complexes may be explained on the basis of chelation theory.Chelation considerably reduces the polarity of the metal ion because of the partial sharing of its positive charge with the donor groups and possible pelectron delocalization over thechelater.Such chelation increases the lipophilic character of the central metal ion,which subsequently favors the permeation through the lipid layer of cell membrane.It is likely that the increased lipo-solubility of the ligand up on metal chelation may contribute to its facile transport into the bacterial cell which blocks the metal binding sides in theenzymesof microorganisms[34]and[35].
On comparing the biological activity for our metal complexes,the following resultsareobtained.
Biological activity against Gram-positive bacteria follows the order:Cu·NTA·leucine=Cu·NTA·valine>Ni·NTA·leucine >Ni·NTA·valine.The biologicalactivity of metal complexes is lower than tetracycline antibacterial agent.
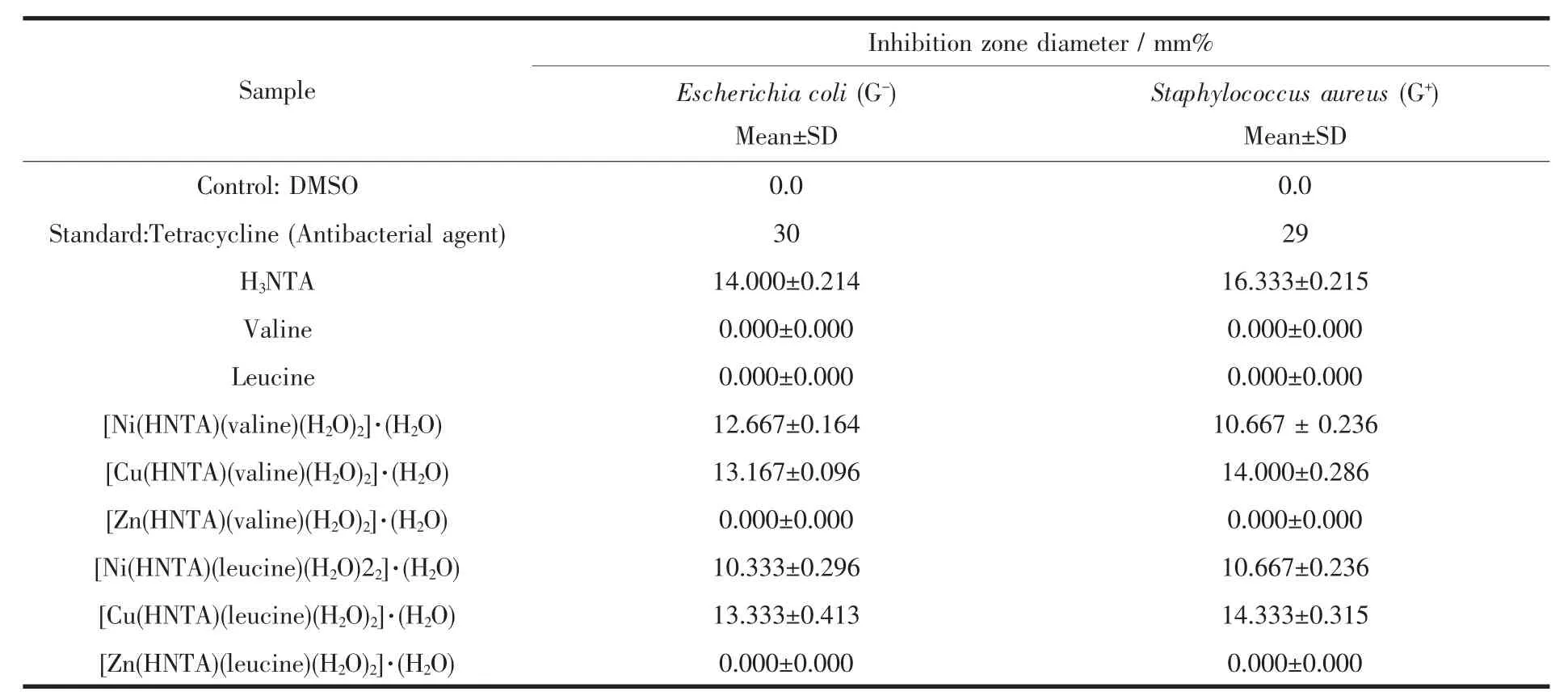
Table 4 Biological activity of NTA,valine and leucine ligands and their metal complexes

Fig.2 a)Percent of inhibition zone diameter(mm·mg-1 sample)relative to standard vs.NTA,leucine and its metal complexes;b)Percent of inhibition zone diameter(mm·mg-1 sample)relative to standard vs.NTA,valine and its metal complexes
Biological activity against Gram-negative bacteria follows the order:Cu·NTA·Leucine>Cu·NTA·valine>Ni·NTA·valine>Ni·NTA·leucine.The biological activity of metal complexes is lower than tetracycline antibacterial agent.
The Biological activity against Candida albicans shows that Ni·NTA·valine causes inhibition zone of 13,12 and 13 mm·mg-1sample.The biological activity of Ni·NTA·valine is lower than amphotericin Bantifungal agent which causes 21,20 and 21mm·mg-1sample inhibition zone.Ni·NTA·valine is the only complex that shows antifungal activity against Candida albicans which makes thiscomplex of interest.
The importance of this lies in the fact that these complexes could be applied fairly in the treatment of somecommon diseasescaused by Escherichia coli,e.g.,septicemia,gastroenteritis,urinary tract infections,and hospital-acquired infections[36-37].It also can be used in treatment of Candida albicans that can cause Candidiasis.Candidiasis encompasses infections that range fromsuperficial,such as oral thrush and vaginitis,tosystemic and potentially life-threateningdiseases.
3 Conclusions
The Ni,Cu,and Zn,nitrilotriacetic acid and valine or leucine ternary complexesprepared in slightly acidic medium have an octahedral structure of the general form [M(HNTA)(valine)(H2O)2]·H2O and[M(HNTA)(leucine)(H2O)2]·H2O,in which nitrilotriacetic acid acts as a tridentate ligand and valine or leucine acts as a monodentate ligand.Two coordinated water molecules and one crystalline water molecule are required to complete octahedral coordination.These complexes behave as dibasic acids and also give enhanced effect in the inhibition zone of Gram-negative and positive bacteria for valine and leucine.
[1]Charlot M F,Kahn O,Jeannin S,et al.Inorg.Chem.,1980,19:1411-1416
[2]Sigel H,Operschall B P,Massoud SS,et al.Dalton Trans.,2006,46:5521-5529
[3]Czakis-Sulikowska D,Czylkowska A,Radwanska D,et al.J.Therm.Anal.Cal.,2007,90:557-564.
[4]Bocarsly JR,Barton JK.Inorg.Chem.,1992,31:2827-2832[5]Farver O,Pecht I.Coord.Chem.Rev.,1989,95:17-23
[6]Crowe J,Dobeli H,Gentz R,et al.Methods Mol.Bio.,1994,31:371-387
[7]Nieba-Axmann SE,Persson A,Hamalainen M,et al.Anal.Biochem.,1997,252:217-222
[8]Maloriery K M,Shnek D R,Sasaki D Y,et al.Chem.Biol.,1996,3:185-192
[9]Bumba L,Tichy M,Dobakova M,et al.J.Struct.Bio.,2005,152:28-35
[10]Hu Q H,Li X F,Du G C,et al.J.Chem.Engin.,2008,143:111-116
[11]Ben Hander G M.Res.J.Chem.Sci.,2012,2(3):12-20
[12]Anderson R L,Bishop W E,Campbell R L.Crit.Re.Toxicol.,1985,15(1):1-102
[13]IARC:Nitrilotriacetic Acid and Its Salts.In:"IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans",IARC,Lyons.1990,48:181-214
[14]Evangelou M W H,Ebel M,Schaeffer A.Chemosphere,2007,68(6):989-1003
[15]Kaur G,Hasan SK,Srivastava R C.Archives of toxicology,1980,45:203-206
[16]Pollack S,Ruocco S.in vivo.Blood.,1981,57(6):1117-1118[17]Mendola M E,Paul T,Strathmann T J,et al.Polyhedron,2009,28:269-78
[18]Kumita H,Jitsukawa K,Masuda H,et al.Inorg.Chim.Acta,1998,283:160-166
[19]Anderegg G,Komplexone X L,Helv.Chim.Acta,1967,50:2333-40
[20]Hopgood D,Augelici R J.J.Am.Chem.Soc.,1968,90:2508-13
[21]Chohan H Z,Arif M,Akhtar M A,et al.Bioinorg.Chem.Appl.,2006,1:83131 DOI:10.1155/BCA/2006/83131
[22]Sakyan I,Logoglu E,Arslan S,et al.Biometals,2004,17(2):115-120
[23]Chohan H Z,Arif M,Sarfraz M.Appl.Organomet.Chem.,2007,21(4):294-302
[24]Ramakrishna R,Ashis K,Patra P,et al.Polyhedron,2008,27:1343-1352
[25]Jian fang D,Lianzhi L,Guihua L,et al.J.Mol.Struc.,2011,986:57-63
[26]Jian L,Tingting L,Sulan C,et al.J.Inorg.Biochem.,2006,100:1888-1896
[27]Khalil M,Hamed E,Abdel Azim S et al.J.Therm.Anal.Calorim.,2010,101:129-135
[28]Dawson R M C.Data for Biochemical Research,Oxford:Clarendon Press,1959.
[29]Santi E,Torre M H,Kremer E,et al.J.Vib.Spectrosc.,1993,5:285-93
[30]Ismail E H,Souaya E R,Amr A M.J.Appl.Poly.Sci.,2012,124(3):1976-1980
[31]Souaya E R,Ismail E H,Mohamed A A,et al.J.Therm.Anal.Calorim.,2009,95:553-558
[32]Gabbott P. Principles and Applications of Thermal Analysis.1st ed.UK:Blackwell Publishing Ltd.,2008.
[33]Shengli J,Mian J,Sanping C,et al.J.Therm.Anal.Calorim.,2001,66:423-429.
[34]Chartone-Souza E,Loyola T L,Rodriguez M B,et al.J.Inorg.Biochem.,2005,99:1001-1008
[35]Gordon A S,Howell L D,Harwood V C.J.Microbiol.,1994,40:408-411
[36]Jawetz E,Melnick J L,Adelberg E A.Review of Medical Microbiology,16th Ed.,Los Anglos,CA:Lang Medical Publications1979.
[37]Hughes W H,Stewart H C.Concise Antibiotic Treatment Butter Worth,London 1970.
