Mass Transfer of Copper(II) in Hollow Fiber Renewal Liquid Membrane with Different Carriers*
2012-10-31ZHANGWeidong张卫东CUIChunhua崔春花andYANGYanqiang杨彦强
ZHANG Weidong (张卫东)**, CUI Chunhua (崔春花) and YANG Yanqiang (杨彦强)
State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China
Mass Transfer of Copper(II) in Hollow Fiber Renewal Liquid Membrane with Different Carriers*
ZHANG Weidong (张卫东)**, CUI Chunhua (崔春花) and YANG Yanqiang (杨彦强)
State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China
The extraction ability of organophosphorus extractant D2EHPA (di-2-ethylhexyl phosphoric acid) and hydroximic extractant Lix984N are investigated by the extraction equilibrium experiments. Effects of carrier concentration and organic/aqueous volume ratio on the mass transfer of hollow fiber renewal liquid membrane (HFRLM)are studied. Results show that, in the extracting process, kerosene and n-heptane are more suitable than methyl-isobutyl ketone, butylacetate and benzene as the diluents of D2EHPA or Lix984N. The favorable feed pH is 4.4 for D2EHPA and 2.6 for Lix984N. The mass transfer flux of HFRLM increases with carrier concentration and finally reaches a plateau. The mass transfer flux and the overall transfer coefficient increase with the organic/aqueous volume ratio,reach the maximum and then decrease.
mass transfer, copper(II), di-2-ethylhexyl phosphoric acid (D2EHPA), Lix984N, carrier
1 INTRODUCTION
Heavy metals exist in many effluents discharged from hydrometallurgical and chemical processes [1]. It is necessary to remove and recover these toxic metals for health/economic/environmental reasons [2]. Many methods, such as reverse osmosis [3], ion exchange,and adsorption, have been reported for dilute wastewater treatment. However, some problems, such as low or no selectivity, high investment or operation costs, and membrane fouling, remain unsolved.
Liquid membranes (LMs), proposed in the 1960s[4], is an efficient and cost-effective method for heavy metal removal and recovery, characterized by simultaneous extraction and stripping processes in the same stage (inner-coupling), non-equilibrium mass transfer,“up-hill” effect and high selectivity. However, for a long time, the instability of supported liquid membrane is still a problem. Hollow fiber flowing liquid membrane [5] and hollow fiber contained liquid membrane [6], proposed at the end of 1980s, overcome the loss and leakage problems in conventional LMs, and avoid the emulsification and de-emulsification steps of emulsion liquid membrane, but the mass transfer resistance increases, due to thicker layers of solvent phase and membrane phase.
Zhang et al. [7, 8] proposed a new liquid membrane technique, hollow fiber renewal liquid membrane (HFRLM), which significantly improves the performance of liquid membrane system. In order to industrialize the HFRLM technology, a detailed study with different carriers is carried out in this study.
It has been proved that organophosphorus extractant D2EHPA (di-2-ethylhexyl phosphoric acid)and hydroximic extractant Lix984N are effective extractant for copper(II). Their different functional groups for complexation with copper(II) result in different extraction mechanisms. They are chosen for study in this work. In the extraction equilibrium experiments,D2EHPA/kerosene and Lix984N/kerosene are used as extractants in simulated wastewater with Cu(II), and H2SO4is used as stripping phase. In the HFRLM experiments, the effects of carrier concentration and organic/aqueous volume ratio on the mass transfer are investigated.
2 EXPERIMENTAL
2.1 Reagents and apparatus
Di-2-ethylhexyl phosphoric acid (D2EHPA), from Tianjin Guangfu Chemical Co. Ltd., was of chemical reagent quality with a purity >98.5%. Lix984N, from Cognis Co., was a mixture of Lix860N and Lix84 with 1︰1 (volume ratio). Commercial kerosene, from Tianjin Damao Chemical Reagent Plant, was washed twice with 20% (by volume.) H2SO4to remove aromatics and then with deionized water three times.Copper sulfate anhydrous, from Shanghai Tingxin Chemical Reagent Plant, was an analytical grade reagent with a purity >99.0%. All other chemicals were of analytical grade and without further purification.
The feed phase, used for D2EHPA, was prepared by dissolving a weighed amount of CuSO4in acetate buffer media, in which the pH value was adjusted as suggested by Komasawa and Otake [9]. The feed phase, used for Lix984N, was prepared by dissolving a weighed amount of CuSO4in deionized water and the pH value was adjusted with H2SO4without buffer.
The hollow fiber module was self-manufactured with small laboratory scale versions specifically designed for the experimental purpose. The polyvinylidenefluoride (PVDF) hollow fibers were from Tianjin Polytechnic University. Additional information about this module is provided in Table 1.
2.2 Experimental procedures
In the experiments of HFRLM, D2EHPA/kerosene and Lix984N/kerosene are used as liquid membrane phase. The hydrophobic fibers are pre-wetted with organic phase more than 10 h for the pores of fibers to be fully filled with organic phase. The stirred mixture of the stripping phase and organic phase is pumped through the lumen side of the module and the feed phase through the shell side in counter-current. The pressures on both sides are controlled by the flow velocity to make a lightly positive pressure on the shell side, in order to prevent the membrane liquid leakage from membrane pores, so that a stable interface is obtained. The experimental process flow diagram is available in our previous work [8].
2.3 Analysis
Once a stable state achieved, aqueous samples are taken from the outlet of lumen and shell sides at preset time intervals. The copper(II) concentration of aqueous phase is analyzed with sodium diethyldithiocabamate spectrophotometric method (GB 7474-87,China). A digital precision ionometer (model PXS-450)with a combined glass electrode is used for pH measurements (±0.01 pH).
The mass transfer coefficient is calculated by the following equation:
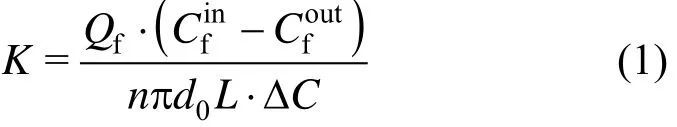
where
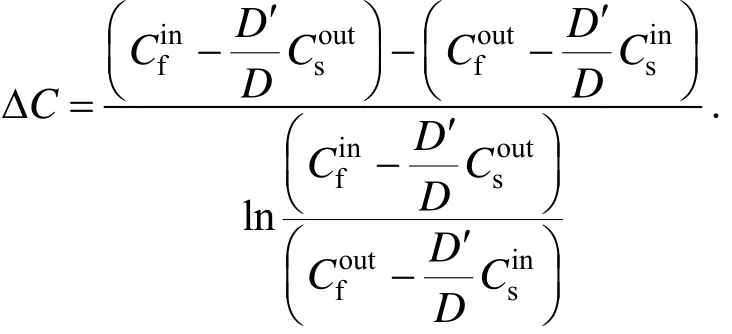
K is the overall mass transfer coefficient, m·s−1; Qfis the flux of feed phase,andare copper(II) concentrations at the inlet and outlet of feed phase and stripping phase, g·m−3, respectively; D and D′ are the distribution coefficient of extraction and back-extraction, respectively.
The mass transfer flux based on the feed phase can be calculated by
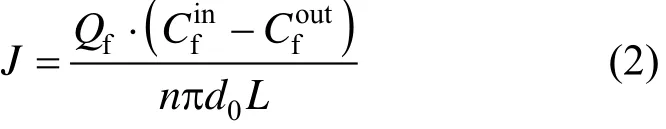
where J is the mass transfer flux based on the feed phase, g·m−2·h−1.
3 RESULTS AND DISCUSSION
3.1 Distribution equilibrium
In order to better understand the mass transfer mechanism in HFRLM, and therefore to determine the optimal operating condition, liquid-liquid extraction equilibrium experiments are conducted, especially for different diluents and feed pH values.
The extractive performances of the D2EHPA system and the Lix984N system are tested using various diluents at the initial feed Cu(II) concentration 70 mg·L−1, with the feed pH value 3.52 for D2EHPA and 1.00 for Lix984N as extractants. The diluents used in these experiments include kerosene, n-heptane, methyl-isobutyl ketone (MIBK), butylacetate and benzene. The results are listed in Table 2. The distribution coefficient is higher when kerosene and n-heptane are used as diluents, while MIBK, butylacetate and benzene give poor performance as diluents, especially for Lix984N.

Table 1 Configuration parameters of hollow fiber membrane and membrane contactor
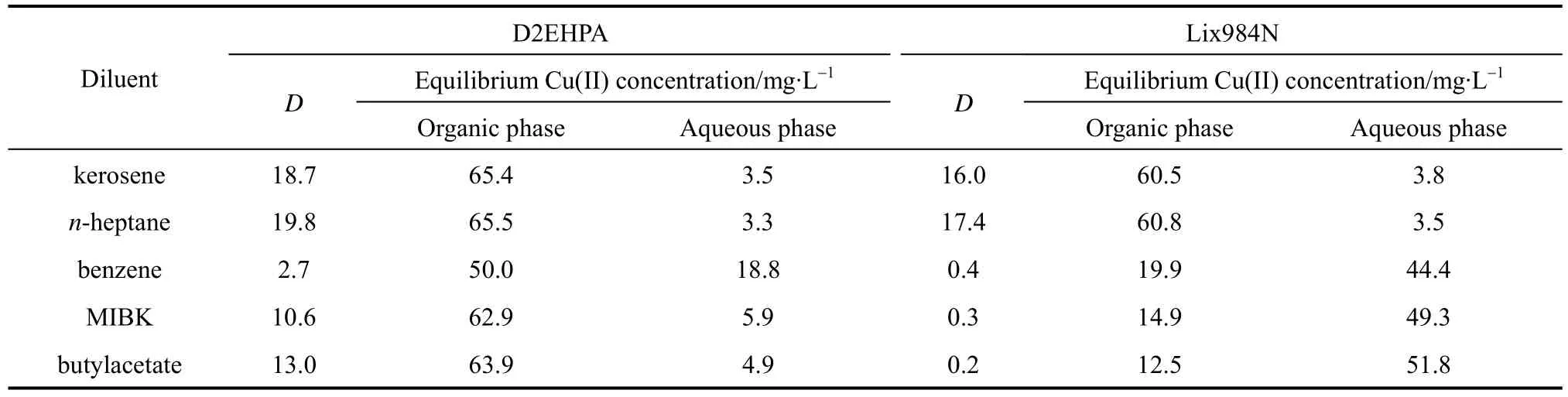
Table 2 Effect of diluents on the distribution coefficient at [D2EHPA] 10% (by volume),[Lix984N] 10% (by volume), and T 293.2 K
It is reported that diluents affects the extraction behavior of the extractant significantly, although they have no extraction ability to metal ions [10]. The solvation effect between diluent and extractant varies with the nature of diluent. The polarity of diluent influences its solvation effect. The stronger the polarity, the stronger the diluent solvation effect, which suggests that the effective concentration of extractant may be reduced and the distribution coefficient decreases. In the following experiments kerosene is used as diluent.
The feed pH is a key variable as most of researchers have mentioned [11, 12]. The extraction equilibrium experiments with 10%(by volume) extractant concentration (D2EHPA or Lix984N) dissolved in kerosene are conducted at the initial feed Cu(II) concentration of 70 mg·L−1. The result is shown in Table 3.
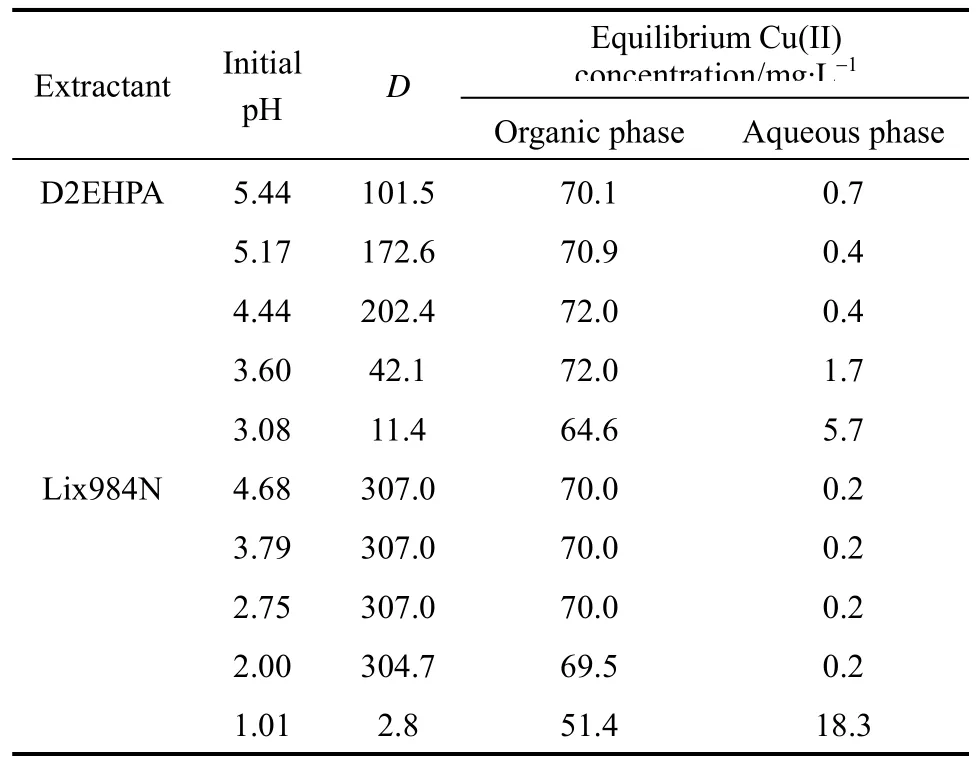
Table 3 Effect of feed pH on distribution coefficient at[D2EHPA]=10%, [Lix984N]10%, Cf0=70 mg·L−1,and T=293.2 K
With D2EHPA/kerosene as extractant, the distribution coefficient increases with the initial feed pH from 3.00 to 4.44, achieves a maximum value at pH of 4.44, and then decreases. The result of D2EHPA agrees well with our previous studies conducted at a higher feed concentration [13].
With Lix984N/kerosene as extractant, the distribution coefficient increases with the initial feed pH,and reaches a plateau at pH of 2.00. Lix984N, as a chelating extractant with acidic groups OH, reacts with copper(II), forms Cu-Lix984N chelates and releases H+. However, besides the acidic groups, some neutral radicals also participate the chelating reaction,forming a stable chelate structure. As a result, the reaction equilibrium is independence of pH value, although the acidity affects the extraction reaction.Therefore, when using Lix984N as extractant, high extraction capacity can be maintained in a wide pH range of 2.0-4.7.
In the following experiments, the initial feed pH value of 4.4 for D2EHPA and 2.6 for Lix984N are used.
3.2 Effect of carrier concentration on HFRLM mass transfer
Prior to the experiments, hollow fibers within the module are wetted with the organic phase at different carrier concentrations for no less than 10 h, for the pores of the fibers to be fully filled with organic phase.The experiments with varying carrier concentrations(Lix984N or D2EHPA) in kerosene are carried out in single-pass mode. The stirred mixture of liquid membrane phase and the stripping phase [2.0 mol·L−1H2SO4, 1︰20 (volume ratio)] flows through the lumen side at the velocity (ut) of 3.0×10−3m·s−1. The feed phase flows through the shell side of the module at the velocity (us) of 0.7×10−3m·s−1. The result is shown in Fig. 1. The experiments are also conducted at different initial feed concentrations, using Lix984N as carrier, and the result is shown in Fig. 2.

Figure 1 Effect of carrier concentration on mass transfer flux at Cf0 70 mg·L−1, pH[D2EHPA]=4.44, pH[Lix984N] 2.60,Vo/Vw 0.1, ut 3.0×10−3 m·s−1, us=0.7×10−3 m·s−1, and T 293.2 K■ D2EHPA; ▼ Lix984N
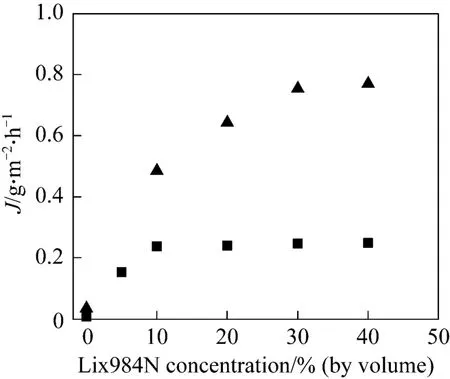
Figure 2 Effect of carrier (Lix984N) concentration on mass transfer flux with different initial feed concentrations at pH 2.60, Vo/Vw 0.1, ut=3.0×10−3 m·s−1, us 0.7×10−3 m·s−1, and T 293.2 K
As shown in Fig. 1, there is almost no mass transfer at the carrier concentration of zero. This is due to the fact that the kerosene can not complex with copper(II), which means that there is no facilitated transport. The mass transfer flux increases with carrier concentration and reaches a plateau at 10% (by volume)carrier concentration, either for D2EHPA or Lix984N,because the mass transfer flux of the HFRLM process depends on the facilitated transport capacity of the carrier in the organic phase. With the increase of carrier concentration, the driving force for mass transfer(distribution coefficient) increases, which will enhance the rate of complex reaction between the organic phase and copper(II) in the feed phase. Therefore, the mass transfer flux is higher with higher carrier concentration. However, when the carrier concentration is too high, the viscosity of the membrane phase increases and the transfer flux is controlled by the diffusion rate of the carrier-metal complex [14].
As shown in Fig. 2, the flux reaches the plateau at 10% (by volume) carrier concentration for 30 mg·L−1and 30% (by volume) carrier concentration for 160 mg·L−1. Also, the transfer flux is lower with lower initial feed concentration. This can be attributed to the mass transfer process being controlled by the diffusion of mental in feed side [15].
3.3 Effect of organic/aqueous ratio on HFRLM mass transfer
The organic/aqueous (o/w) volume ratio in the lumen side has great influence on the mass transfer of the hollow fiber renewal liquid membrane process by affecting the flowing liquid membrane and the renewal rate. The experiments are carried out in the Copper(II)-Lix984N system and D2EHPA-2.0 mol·L−1H2SO4system with varying o/w volume ratios in the range of 0-0.15. The result is shown in Fig. 3. Both mass transfer flux and the overall mass transfer coefficient increase with the o/w volume ratio, and reach the maximum at o/w volume ratio of 0.05-0.10, and then decease.
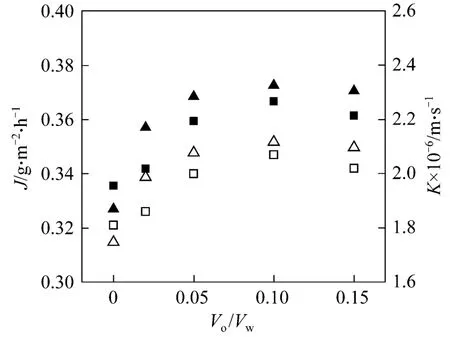
Figure 3 Effect of o/w volume ratio on mass transfer at Cf0 70 mg·L−1, pH[Lix984N]=2.60, pH[D2EHPA]=4.44, [D2EHPA]10%, [Lix984N]=10%, ut=2.9×10−3 m·s−1, us 0.6×10−3 m·s−1, and T=293.2 K■ JLix984N; ▲ JD2EHPA; □ KLix984N; △ KD2EHPA
With the o/w volume ratio of 0, the mass transfer process becomes a hollow fiber supported liquid membrane (HFSLM) process. The mass transfer flux and the overall mass transfer coefficient are smaller compared with HFRLM process. There is no organic droplet on the surface of the lumen side, which means no renewal of liquid membrane. Since the operation conditions are similar, the resistances of LM phase and shell side will keep same, and the difference of overall mass transfer coefficient between HFSLM and HFRLM is contributed to lumen side. It indicates that with the renewal effect of HFRLM, the small droplet of organic phase will contact with the lumen stripping bulk, and therefore enlarge the phase contact area. On the other hand, the random collision of droplets provides the surface renewal and increases the mass transfer rate. The result also proves that HFRLM has better mass transfer behavior compared to HFSLM,which makes it a potential technology of LMs.
With higher renewal rate resulted from increasing the o/w volume ratio, the individual resistance will be reduced further, so that both mass transfer flux and the overall mass transfer coefficient increase. However,when the o/w volume ratio is too large, the thickness of the flowing liquid membrane in the lumen side of the fibers will get thicker, which increases the mass transfer resistance in liquid membrane phase. Also,higher o/w volume ratio means more organic carrier being consumed. Thus, there is a favorable o/w volume ratio in the range of 0.05-0.10.
ACKNOWLEDGEMENT
We are grateful to Prof. Xiaolong Lü of Tianjin Polytechnic University for supplying us with PVDF hollow fibers.
1 Wassink, B.D., Howard, J., “Solvent extraction separation of zinc and cadmium from nickel and cobalt using Aliquat 336, a strong base anion exchanger, in the chloride and thiocyanate forms”, Hydrometallurgy, 57, 235-252 (2000).
2 Kurniawan, T.A., Chan, G.Y.S., Lo, W.H., Babel, S., “Physico-chemical treatment techniques for wastewater laden with heavy metals”, Chem. Eng. J., 118, 83-98 (2006).
3 Liu, F.N., Zhang, G.L., Meng, Q., Zhang, H.Z., “Performance of nanofiltration and reverse osmosis membranes in metal effluent treatment”, Chin. J. Chem. Eng., 16 (3), 441-445 (2008).
4 Li, N.N., “Separating hydrocarbons with liquid membranes”, U.S.Pat., 3410794 (1968).
5 Teramoto, M., Matsuyama, H., Yamashiro, T., “Separation of ethylene from ethane by a flowing liquid membrane using silver nitrate as a carrier”, J. Membr. Sci., 46, 115-136 (1989b).
6 Sengupta, A., Basu, R., Sirkar, K.K., “Separation of solutes from aqueous solution by contained liquid membrane”, AIChE J., 34,1698-1798 (1988).
7 Zhang, W.D., Li, A.M., Li, X.M., Ren, Z.Q., “Principle of liquid membrane and hollow fiber renewal liquid membrane”, Modern Chemical Industry, 25, 66-68 (2005). (in Chinese)
8 Ren, Z.Q., Zhang, W.D., Liu, Y.M., Dai, Y., Cui, C.H., “New liquid membrane technology for simultaneous extraction and stripping of copper(II) from wastewater”, Chem. Eng. Sci., 62, 6090-6101 (2007).
9 Komasawa, I., Otake, T., “Kinetic studies of the extraction of divalent metals from nitrate media with bis(2-ethylhexyl)phosphoric acid”, Ind. Eng. Chem. Fundam., 22, 367-371 (1983).
10 Mohapatra, D., Kim, H.I., Nam, C.W., Park, K.H., “Liquid-liquid extraction of aluminium(III) from mixed sulphate solutions using sodium salts of Cyanex 272 and D2EHPA”, Sep. Purif. Technol., 56,311-318 (2007).
11 Zhu, Z.Z., Hao, Z.L, Shen, Z.S., Chen, J., “Modified modeling of the effect of pH and viscosity on the mass transfer in hydrophobic hollow fiber membrane contactors”, J. Membr. Sci., 250, 269-276 (2005).
12 Gherrou, A., Kerdjoudj, H., Molinari, R., Drioli, E., “Removal of silver and copper ions from acidic thiourea solutions with a supported liquid membrane containing D2EHPA as carrier”, Sep. Purif.Technol., 28, 235-244 (2002).
13 Ren, Z.Q., Zhang, W.D., Meng, H.L., Liu, Y.M., Dai, Y., “Extraction equilibria of copper(II) with D2EHPA in kerosene from aqueous solutions in acetate buffer media”, J. Chem. Eng. Data, 52, 438-441(2007).
14 Shukla, J.P., Sonawane, J.V., Kumar, A., Singh, R.K., “Amine facilitated up-hill transport of plutonium(IV) cation across an immobilized liquid membrane”, Indian J. Chem. Technol., 3, 145-148(1996).
15 Alguacil, F.J., Alonso, M., “Description of transport mechanism during the elimination of copper(II) from wastewaters using supported liquid membranes and Acorga M5640 as carrier”, Environ.Sci. Technol., 39, 2389-2393 (2005).
2009-05-18, accepted 2009-10-16.
* Supported by the Program for New Century Excellent Talents in University (NCET-05-0122) and the National Natural Science Foundation of China (20576008, 20706003).
** To whom correspondence should be addressed. E-mail: zhangwd@mail.buct.edu.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Top-cited Articles in Chemical Engineering in Science Citation Index Expanded: A Bibliometric Analysis
- Flame Imaging in Meso-scale Porous Media Burner Using Electrical Capacitance Tomography*
- Advances in Large-eddy Simulation of Two-phase Combustion (I)LES of Spray Combustion*
- Adsorptive Removal of Para-chlorophenol Using Stratified Tapered Activated Carbon Column
- Analysis of Absorption and Reaction Kinetics in the Oxidation of Organics in Effluents Using a Porous Electrode Ozonator*
- Modelling and Fixed Bed Column Adsorption of Cr(VI) onto Orthophosphoric Acid-activated Lignin
