一锅煮法合成多取代的2-吡咯烷酮
2011-09-18王科伟樊月琴孟双明贾治芳
王科伟,樊月琴,孟双明,郭 永*,冯 锋,贾治芳
(山西大同大学化学与化工学院,山西大同 037009)
一锅煮法合成多取代的2-吡咯烷酮
王科伟,樊月琴,孟双明,郭 永*,冯 锋,贾治芳
(山西大同大学化学与化工学院,山西大同 037009)
通过简单易得的α-乙酰基环丙基酰胺与NH2OH·HCl一锅反应,高效地合成了多取代的2-吡咯烷酮,其机理涉及到一个三元环开环和分子内亲核环合反应。对它们的结构用核磁共振氢谱、核磁共振碳谱和元素分析进行了表征。
2-吡咯烷酮;环丙基酰胺;环合反应
2-吡咯烷酮广泛存在于自然界的各种生理活性天然产物中,如它是一种促性腺激素释放激素的主要结构单元[1]。同时2-吡咯烷酮是医药、农药、染料、肽类等化学品的重要原料和中间体。若其作为肽的端链还对化合物的构象起到一个稳定的作用[2-3]。许多多取代的2-吡咯烷酮已经应用在多种药物合成生产中并申请专利[4-5]。
随着小环化合物的发展,环丙烷化学倍受关注,特别是在杂环的合成中,而且这一领域已经成为研究热点。许多杂环化合物如吡咯、唑烷、嘧啶酮、喹啉等的衍生物均可以通过环丙烷类化合物制得[6-9]。我们在对不同的环丙基酰胺类化合物研究的基础上[10-11],设计将环丙基酰胺类化合物开环并实现其关环,期望得到一类新的2-吡咯烷酮类化合物。本文用一锅煮法以一系列α-乙酰基环丙基酰胺类化合物与NH2OH·HCl反应,制得了多取代的2-吡咯烷酮,合成路线见图1。
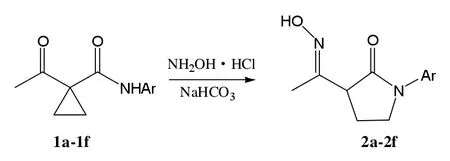
图1 2-吡咯烷酮合成路线
1 实验部分
1.1 仪器与试剂
仪器:VARIAN UNITY-500 MHz核磁共振仪(内标TMS,溶剂CDCl3);MAGNA-IR 560型红外光谱仪(KBr压片法);Agilient 1100 LCMsD型质谱仪;PE-2400自动元素分析仪;旋片式真空泵;三用紫外分析仪;79-1型磁力加热搅拌器;高效薄层板;柱层析分离柱。
药品:各种必需药品均为分析纯试剂,液体试剂用前经无水处理,重蒸备用.
1.2 3-(1-甲基酮肟基)-N-苯基-2-吡咯烷酮的合成
称取乙酰乙酰苯胺(100mmol17.7)于500mL的圆底烧瓶中,加入150mL DMF溶液,搅拌5min,加入220mmol无水K2CO3,搅拌1h后,加入120mmol BrCH2CH2Br于反应器内反应,TLC监测,10h后反应完毕。将反应溶液分批倒入冰水中,充分搅拌,抽滤,水洗,自然晾干,得白色粉末状固体1a 18.9g,产率为93%。同样的方法合成1b-1f.
称取1a 1-乙酰基-N-苯基环丙基酰胺(2.03g,10mmol)和NaOAc(1.64g,20mmol)于50mL的圆底烧瓶中,加入25mL无水乙醇和1mL水,搅拌,加入NH2OH·HCl(1.39g,20mmol),加热回流。TLC监测,大约5.0h结束。将体系倒入饱和氯化钠溶液中,先用饱和NaHCO3溶液调节pH值到中性,用30mL CH2Cl2萃取3次,干燥,柱层析分离,得白色固体1.86g,产率为85%。经核磁表征是2a,见图2。
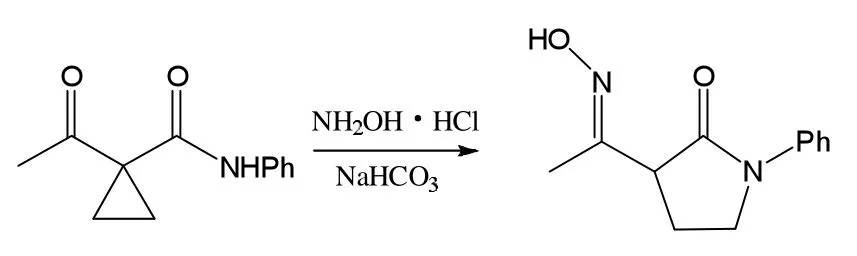
图2 2a制备
同样的方法制的2b-2f.
目标化合物的性状、产率、熔点、核磁共振谱图、与元素分析数据如下:
2a:白色固体,产率85%,熔点115~118℃;1H NMR(CDCl3,500MHz)δ=2.10~2.21(m,2H),2.35(m,1H),2.93(S,3H),3.75~3.78(m,1H),3.79~3.81(m,1H),7.28(d,J=7.5,2H),7.42(m,3H),8.15(br,1H);13C NMR(125MHz,CDCl3)δ=12.11,20.62,49.65,52.49,119.57,128.11,128.96,164.63,170.87。C12H14N2O2理论值:C,66.04;H,6.47;N,12.84。实测值:C,66.25;H,6.55;N,12.70。
2b:白色固体,产率81%,熔点121~123℃;1H NMR(CDCl3,500MHz):δ=1.99~2.09(m,4H),2.35(s,1H),3.52~3.56(m,1H),3.75~3.84(m,5H),7.97(d,J=7.5,2H),7.27(m,2H),8.15(br,1H);13C NMR(125MHz,CDCl3)δ=11.96,23.46,48.18,48.31,55.62,112.04,120.85,126.91,128.49,128.86,154.72,155.94,172.73。C13H16N2O2理论值:C,67.22;H,6.94;N,12.06。实测值:C,67.35;H,6.85;N,12.32。
2c:白色固体,产率73%,熔点128~132℃;1H NMR(CDCl3,500MHz)δ=2.03(s,3H),2.33~2.36(m,2H),3.53~3.56(m,1H),3.73~3.83(m,5H),6.95~6.99(m,2H),7.24~7.28(m,2H);13C NMR(125 MHz,CDCl3)δ=12.16,15.42,23.65,48.35,48.49,55.81,112.24,121.08,127.11,128.68,129.04,154.92,156.19,172.86。C13H16N2O3理论值:C,62.89;H,6.50;N,11.28。实测值:C,62.98;H,6.75;N,10.32。
2d:白色固体,产率90%,熔点117~119℃;1H NMR(CDCl3,500MHz)δ=2.02(s,3H),2.31~2.35(m,5H),3.50~3.54(m,1H),3.79~3.88(m,2H),7.17(d,J=8.0,2H),7.49(d,J=8.0,2H),7.99(s,1H);13C NMR(125MHz,CDCl3)δ=12.56,21.01,22.65,47.25,49.88,120.22,129.55,134.72,136.82,155.97,171.83。C13H16N2O2理论值:C,67.22;H,6.94;N,12.06。实测值:C,67.45;H,6.75;N,12.12。
2e:白色固体,产率89%,熔点120~122℃;1H NMR(CDCl3,500MHz)δ=2.00(s,3H),2.29~2.34(m,2H),3.48~3.51(m,1H),3.77~3.84(m,5H),6.88(d,J=8.0,2H),7.49(d,J=8.0,2H),7.58(s,1H);13C NMR(125MHz,CDCl3)δ=12.55,22.70,47.54,49.72,55.64,114.23,121.99,132.51,155.94,156.95,171.75。C13H16N2O3理论值:C,62.89;H,6.50;N,11.28。实测值:C,62.76;H,6.45;N,11.44。
2f:白色固体,产率71%,熔点98~101℃;1H NMR(CDCl3,500MHz):δ=2.03(s,3H),2.05~2.08(m,1H),2.19~2.20(m,4H),2.31~2.37(m,5H),3.48~3.52(m,2H),3.67~3.70(m,2H),7.02(s,2H),7.08(s,1H);13C NMR(125MHz,CDCl3)δ=12.61,17.92,20.65,21.63,49.99,52.47,121.45,126.23,131.12,134.28,135.65,143.48,164.62,170.87。C14H18N2O2理论值:C,68.27;H,7.37;N,11.37。实测值:C,68.33;H,7.45;N,11.49。
2 结果与讨论
本研究中的关键问题是,根据以前的文献得知环丙基类化合物的开环大都在酸性条件下进行,在碱性条件下实现开环的研究比较少,于是我们做了如上实验。首先是NH2OH·HCl的量,我们试图用1倍量的NH2OH·HCl去实现开环,虽能得到目标产物,但产率较低,只有将NH2OH·HCl的量控制在2倍的时候才能得到满意的结果。有趣的是我们在反应中检测到了3-乙酰基-N-(4-甲基苯基)-2-吡咯烷酮,并由1H NMR、13C NMR确认,其核磁数据如下:1H NMR(CDCl3,500MHz)δ=2.15~2.19(m,1H),2.33(s,3H),2.46(s,3H),2.64~2.68(m,1H),3.75~3.79(m,2H),3.85~3.90(m,1H),7.16(d,J=7.5,2H),7.43(d,J=7.5,2H);13C NMR(125MHz,CDCl3)δ=19.77,21.03,30.09,47.46,57.26,120.46,129.58,135.02,136.53,168.98,203.50。其中有酮羰基的特征峰203.50。根据我们所得的结果和先前我们的研究结果[10-11],提出了一个可能的机理,见图3。
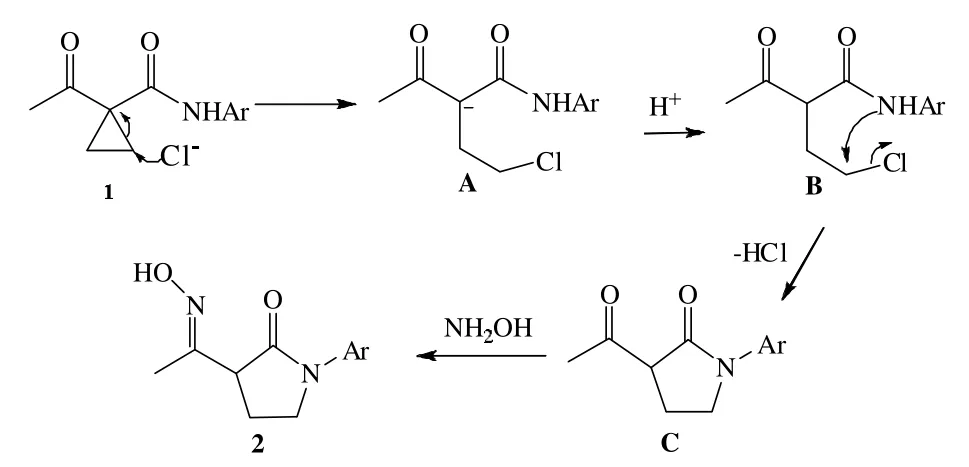
图3 制备机理
[1]Sievertsson H,Chang J K,Bgentoft C,et al.Synthesis of the luteinizing releasing hormone of the hypothalamus and its hormonal activity[J].Biochem Biophys Res Commun,1971,42,1180-1184.
[2]Freidinger Rm,Perlow D S,Veber D F.Protected lactam-bridged dipeptides for use as conformational constraints in peptides[J].J Org Chem,1982:47,104-109.
[3]Bell Im,Gallicchio S N,Abramsm,et al.Design and Biological Activity of(S)-4-(5-{[1-(3-Chloro benzyl)-2-oxopyrrolidin-3-ylamino]methyl}imidazol-1-ylmethyl)benzonitrile,a 3-Aminopyrrolidinone Farnesyl-transfer-ase Inhibitor with Excellent Cell Potency[J].J Med Chem,2001,44:2933-2949.
[4]Dominguez C,Chen G,Xi N,et al.PCP Int Appl WO:0144230 A1[P].20010621.
[5]Ishihara Y,Imamura S,Hashiguchi S,et al.Pat Appl,EP:1180513 A1[P].20020220.
[6]Zhang Z,Zhang Q,Sun S,et al.Domino Ring-Opening/Recyclization Reactions of Doubly Activated yclopropanes as a Strategy for the Synthesis of Furoquinoline erivatives[J].Angew Chem Int Ed,2007,46:1726-1729.
[7]Xiong T,Zhang Q,Zhang Z,et al.A Divergent Synthesis of γ-Iminolactones,Dihydroquinolin-2-ones,and γ-Lactames from β-Hydroxymethyly clopropanylamides[J].J Org Chem,2007,72:8005-8009.
[8]Schmalz H,Zhang J.Gold(I)-Catalyzed Reaction of 1-(1-Alkynyl)-cyclopropyl Ketones with Nucleophiles:A Modular Entry to Highly Substituted Furans[J].Angew Chem Int Ed,2006,45:6704-6707.
[9]Shim,Tang X,Yang Y.Lewis Acid Mediated Reactions of 1-Cyclopropyl-2-arylethanones with Allenic Esters:A Facile Synthetic Protocol for the Preparation of Dihydrofuro[2,3-h]chromen-2-one Derivatives[J].Org Lett,2007,9:4017-4020.
[10]Pan W,Dong D,Wang K,et al.Efficient One-Pot Synthesis of Highly Substituted Pyridin-2(1H)-ones via the Vilsmeier-Haack Reaction of 1-Acetyl,1-Carbamoyl Cyclopropanes[J].Org Lett,2007,9:2421-2423.
[11]Wang K,Xiang D,Liu J,et al;Efficient and Divergent Synthesis of Fully Substituted 1H-Pyrazoles and Isoxazoles from Cyclopropyl Oximes[J].Org Lett,2008,10:1691-1694.
〔编辑 杨德兵〕
Efficient One-Pot Synthesis of Highly Substituted Pyrrolidin-2-ones
WANG Ke-wei,FAN Yue-qin,MENG Shuang-ming,GUO Yong,FENG Feng,JIA Zhi-fang
(Shcool of Chemistry and Chemical Engineering,Shanxi Datong University,Datong Shanxi,037009)
A facile and efficient one-pot synthesis of highly substituted pyrrolidin-2-ones is developed via the NH2OH·HCl and readily available α-acetyl cyclopropyl amide,and a mechanism involving sequential ring-opening,and intramolecular nucleophilic cyclization reactions is proposed,Their structures were characterized 1H NMR,13C NMR spectra and elemental analysis.
pyrrolidin-2-ones;cyclopropyl amide;cyclization reactions
TQ560.7;O626
A
1674-0874(2011)03-0045-03
2011-04-02
山西省自然科学基金[2010011018]
王科伟(1982-),男,山西晋城人,助教,研究方向:杂环化合物合成方法学;*郭永,男,教授,通信作者。
