Electrophysiologicalresponses of Plutella xylostella to 9 different plant volatiles
2011-06-09XUHongxingWANGSuTANXiaolingZHANGFanLabofNaturalEnemyResearchInstituteofPlantEnvironmentProtectionBeijingAcademyofAgricultureForestrySciencesBeijing00097ChinaCollegeofPlantProtectionNorthwestUniversityYangling7200Shannx
XU Hong-xing,WANG Su,TAN Xiao-ling,2,ZHANG Fan*(.Lab of Natural Enemy Research,Institute of Plant& Environment Protection,Beijing Academy of Agriculture& Forestry Sciences,Beijing 00097,China;2.College of Plant Protection,Northwest A & F University,Yangling 7200,Shannxi Province,China)
1 Introduction
The diamondback moth(DBM),Plutella xylostella(L.)(Lep.,Plutellidae),is one of the most destructive insect pests of crucifers,including various important oilseed and food crops(Talekar and Shelton,1993).The use of Plutella xylostella sex pheromones,attracting the male moths and reducing the population quantity in the field,provided strategies for a pest control alternative to the use of insecticides(Han and Zhang,2001).But the limits of this approach are the fact that P.xylostella sex pheromones can only attract males and leave the female population unaffected,which results in the population of next generation didn't lower even in the presence of a reduced number of males in the fore-generation(Pelosi and Maida,1995).The olfactory system of insects can not only detect pheromone,playing an important role in locating partners,but also respond to plant volatiles in host- finding,oviposition and hibernation behaviors(Kaissling et al.,1987;Larsson et al.2004).
In this study,we designed a plant volatile collection apparatus and collected the volatiles of 5 species of cruciferae plants that could directly attract both meal and female moths and 4 species of non-cruciferae plants,respectively.Electroantennogram(EAG)was also detected to analysis the host specificity and preference of P.xylostella.
2 Materials and methods
2.1 Insect and plants
The P.xylostella adults were collected from cab-bage fields of Wangjiayuan Experimental Station of Beijing Academy of Agriculture and Forestry Sciences,Changping District,Beijing,China.In the laboratory,P.xylostella was reared on cabbage(Brassica Oleracea),at 26±1°C,relative humidity 60%,and 16 h∶8 h(light/dark)photoperiod.
All plants were raised in clear cages(60×45×35 cm,H∶W∶L)separately at room temperature.The injured plants were obtained by releasing 10 P.xylostella larvas and 5 adults to each cage.5 days after releasing,the injured plants can be operated in volatiles collection experiments.The growth status of plants when we collected odours were showed in Tab.1

Tab.1 Growth status of 9 species of plants when volatiles collection
2.2 Collection and elution of Plants volatiles
The method we collected and eluted plants volatiles was based on those of Guo et al.(1997)and some improvements were done to increase efficiency.The schematic diagram of plant volatiles collection apparatus was showed in Fig.1.The links in this apparatus were all sealed by PTFE tape.GDX -101(Tianjin Guangfu Fine Chemical Research Institute)was chosen as adsorbent of plant volatiles(Guo et al.1997),absorbing column(5 cm ×0.8 cm)containing 30 mg GDX-101 were prepared with both ends jammed by glass wool.The flow rate of atmospheric sampling instrument was 100ml/min,and each plant volatile collecting time was 4 hours.
Adsorbent containing in the absorbing column was transferred to the centrifugation minicolumn,add 400μl methylene dichloride to the minicolumn and cap the minicolumn securely.Shake the minicolumn vigorously by hand for 15 seconds and put it into a 1.5 mL centrifuge tube.Incubate for 5 minutes at room temperature and centrifuge the sample at 12,000×g for 3 min.The liquid in centrifuge tube was the mixture containing plant volatiles and stored at 20℃ until use.
2.3 Electroantennography Concentration Respon-ses

Fig.1 Plant volatiles collection apparatus
An antenna from 3-day-old moth was excised at its base before test,and 1mm of the antenna distal part was cut off.The antennal preparation was mounted on PR electrode using Spectra 360 electrode gel 5min before the EAG recording began.10μl test solution was pipetted onto a 3×0.5 cm stirp of filter paper,after which the filter paper was placed inside a 12cm long glass Pasteur pipette.The prepared Pasteur pipette was linked to the stimulus controller during each test.Stimulus air flow rate is 60 mL/min and the continuous air flow rate is 120 mL/min.Test substances and controls were applied(0.5 s pulse)at 30 s intervals separated by continuous air flow.Before and after each sample stimulating,it was necessary to stimulate the antenna using control compound,so as to monitor the activity of antenna and calibrate the error.Each sample was tested 3 times with 5 antennas of males and females.The EAG apparatus and the corresponding software was made of Syntech Ltd.,Hilversum,The Netherlands.
2.4 Statistical analysis
EAGs were compared statistically to different plants,sex and plant status by using ANOVA analysis.The results were assessed by statistic analysis software SPSS 16.0 and the values were calculated as Mean±Se.The one-way ANOVA was executed to analyze the effect to the EAG related values with the independent variables as host plant,sex and plant status.Furthermore,we used general linear model to analyze the cross interaction among all independent variables.
3 Result
tests were competed under microscope monitoring to avoid separation of antenna from PR electrode.The mean EAG relative values of male/female P.xylosstella to healthy/injured plant volatiles were showed in Tab.2.Of all volatiles tested,healthy cabbage volatiles elicited the strongest EAG response to male's antenna.Significantly different between the cruciferae plants and non-cruciferae plants was also showed in Tab.2.By ANOVA analysis,the factors host plant and sex of P.xylostella showed influence the EAGRV significantly(see Tab.3,Fig.2 A,B.).But the plant status showed no significant effect to the variation of EAG-RV(Fig.2 B).Male moth may sense the volatile matters generated by host plant more efficiently than female did.
The interactions of different factors were also tested by multiple-ANOVA analysis process.Even there were not significant differences in both host plant and plant status treatments,the cross effect by these two factors existed obviously.Similarly,the cross effects incorporated with sex factor also showed interactive impacts to the EAG-RV.And in triangle cross effect as host plant× sex × plant status,the joint influence exceed the level of significant.
9 healthy plants volatiles and 5 injured plants volatiles were collected in this study,and all those plants volatiles were stored at-20℃ until use.PR electrode was chosen as the appropriate electrode rather than glass since the antenna of P.xylostella is long enough to conveniently mounted on PR electrode.The EAG
4 Discussion
The olfactory system of insects is a highly specific and sensitive chemical detector,which can detect,recognize and transfer chemical information from the environment and trigger a serial of specific behavioralreactions,such as locating partners,food sources and oviposition and hibernation sites,and to avoid dangerous situations or unsuitable habitats and hosts(Kaissling et al.,1987;Breer et al.,1994).Botanic volatile matters are supposed to many targeted behaviour and physiological process in arthropod which could be related with correlation in the exploration of co-evolution and attack-defense competition system(Hedin et al.,1979;Finch,1986;Hansson et al.,1989).Especially for phytophagous insect,the perception,detection and identification of volatile chemical materials generated by host plant candidate always play fatal roles during their life maintenance(Qin,1987).

Tab.2 Mean EAG relative values of P.xylostella to different plant volatiles
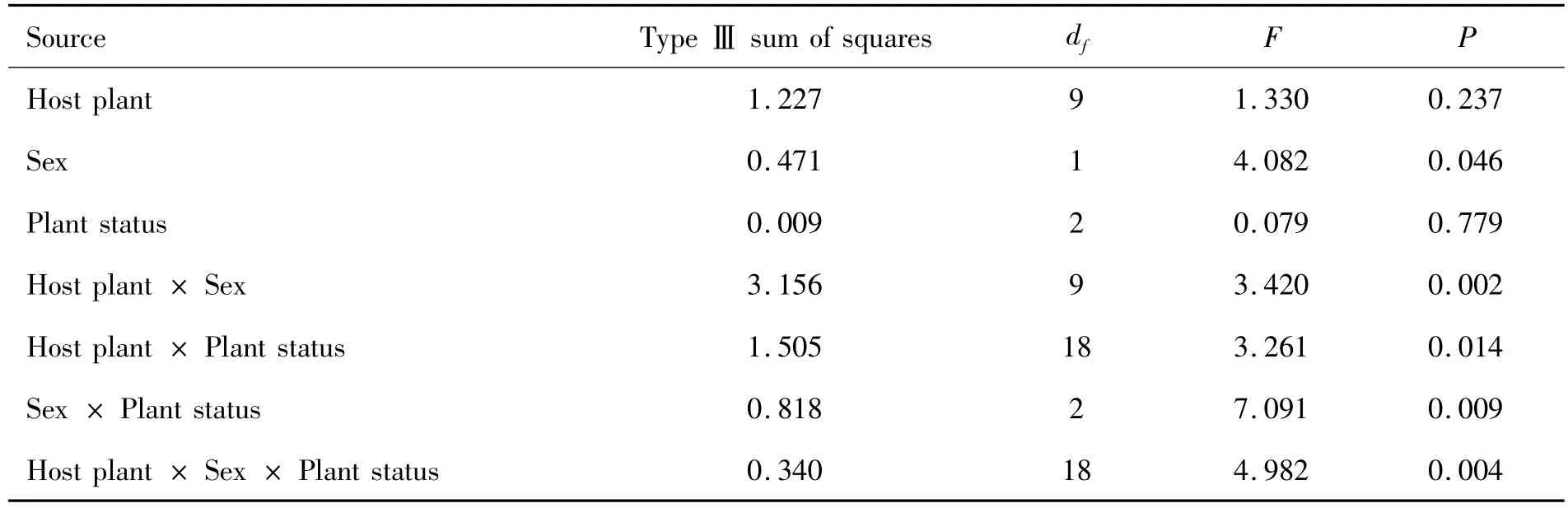
Tab.3 Results of multiple-ANOVA analysis of EAG related value with the in dependent variables as host plant diversity,sex of P.xylostell and host plant status

Fig.2 Mean number(+SE)of EAG related value influenced by three different factors
As well as importance of sex pheromone,the intensive research of general botanic odor could help us to learn more about the host plant preference,host location and population distribution and expansion efficaciously(Zhang et al.,2006).Utilizing of olfactory -meters to test the responses of P.xylostella to host plants was studied in a wide range.This kind of researches indicated the botanic odor will help the moth to find their suitable host in a very short time period and long distance(Han and Zhang,2001;Bertschy et al.,1997).But most related studies just only focused external performance of testee moth without any exploration of in physiological path.This study provides physiological evidence that cruciferae plant volatiles represent significantly higher attract role to P.xylostella than that of non-cruciferae,which can explain the P.xylostella host-plant specifity and preference in physiological path.
It is a extremely complex detecting process for olfactory system of insects to recognized the plant volatiles.There is a great difference of EAG response between the single component and total components stimulating(Zhang et al.,2005;Yang et al.2010).The result of our study compared the healthy and injured plant volatiles initially and the significant difference between them represents some specific compounds induced by P.xylostella have phobotaxis - like effect.So the study of plant volatiles component and its concentration need to be carried out in the future.
Acknowledgements
Present study was supported by National Natural Science Foundation of China(31071731)and the Knowledge Innovation Project of Chinese Academy of Sciences-Important Direction Projects(KSCX2-YW-N-42-04).We thank Zhang YP(college student of Changjiang University)for her first-phase preparations during the summer internship.
Bertschy C,Turlings TCJ,Bellotti AC,Dorn S,1997.Chemically-mediated attraction of three parasitoid species to mealybug - infested cassava leaves.Florida Entomologist,80(3):383-395.
Breer H,Raming K,Krieger J,1994.Signal recognition and transduction in olfactory neurons.Biochim.&Biophys.Acta,1224,277-287.
Field LM,Pickett JA,Wadhams LJ,2000.Molecular studies in insect olfaction.Insect Mol.Biol.,9(6),545 -551.
Finch S,1986.Assessing host- plant finding by insects.In:Miller JR,Miller TA eds.Insect Plant Interaction.New York:SpringerVerlag Press.23- 631.
Guo XF,Yin HW ,Yan SC,HuZJ,Guo TQ,1997.Sampling and analyzing of volatile odorant of plant.Journal of Northeast Forestry University,25(5):105-108.
Han BY,Zhang ZN,2001.Chemical ecology of the diamond-back moth,Plutella xylostella:progress and prospect.Entomological knowledge,38(3):177 -181.
Hansson BS,Van Der Pers JNC,Lofquist J,1989.Comparison of male and female olfactory cell response to pheromone compounds and plant volatiles in turnip moth,Agrotis segetum.Physiol.Entomol.,14:147 -1551
Hedin PA,Mckibben GH,Mitchell EB,JohnsonWL,1979.I-dentification and field evaluation of the compounds comprising the sex pheromone of the female - bollweevil.J.Chem.Ecol.,5(4):617 -628.
Kaissling KE.,Zack SC, Rumbo ER.1987. Adaptation processes in insect olfactory receptors:mechanisms and behavioral significance.Ann.N.Y.Acad.Sci.,510:104-112.
Larsson MC,Domingos AI,Jones WD,Chiappe ME,Amrein H,Vosshall LB,2004.Or83b encodes abroadly expressed odorant receptor essential for Drosophila olfaction.Neuron,43(5):703-714.
Pelosi P,Maida R,1995.Odorant- binding proteins in insects.Com.Biochem.Physiol.B:Biochem.Mol.Biol.,111(3):503-514.
Qin JD,1987.The Relationship Between Insects and Plants.Beijing:Science Press.25 -37.
Talekar NS,Shelton AM,1993.Biology,ecology and management of the diamondback moth.Annu.Rev.Entomol.,38:275-301.
Tirard A,Renucci M,Provost E,Khlat J,Clement JL,2002.Are polyamines involved in olfaction?An EAG and biochemical study in Periplaneta Americana antennae.Chemical Senses,27:417 -453.
Yang H,Yang MF,Yang W,Yang CP,Zhu TH,Huang Q,Zhao XY,2010.Behavioral and EAG responses of Cyrtotrachelus buqueti Guerin-Meneville(Coleoptera:Curculion idae)adults to host volatiles and their body extracts.Acta Entanologica Sina,53(3):286-292.
Zhang TX,Cui WZ,Sun XG,Zhang WG,Liang ZG,2005.Electmantennogram responsesofAcantholyda posticalis Matsumura to volatiles of different trees.Acta Entomol.,48(4):514-5171
Zhang ZC,Wang MQ,Wang N,Li J,Zhang GA,2006.Olfactory responses of Plutella xylotella to polyamines.Acta Entomoligica Sinica,49(1):142-145
杂志排行
环境昆虫学报的其它文章
- 香蕉园内红火蚁种群时序动态规律研究
- 深圳市红火蚁种群动态的初步研究
- 祁连山东大河林区天敌昆虫区系结构研究
- Preferential feeding of an anthocorid predator Blaptostethus pallescens Poppius on different stages of cotton mealybug
- Quality assessment of local Trichogramma species(Hymenoptera,Trichogrammatidae)to control the olive moth Prays oleae Bern.(Lepidoptera,Yponomeutidae)
- Determination and evaluation on field control efficacy of Trichogramma dendrolimi against Ostrinia furnacalis
