支撑液膜萃取及其在食品安全检测中的应用
2010-10-27项锦欣刘洵妤1赵国华1
刘 嘉,项锦欣,刘洵妤1,,赵国华1,,*
(1.西南大学食品科学学院,重庆 400715;2.重庆市农产品加工技术重点实验室,重庆 400715;3.重庆理工大学化学与生物工程科学学院,重庆 400050)
支撑液膜萃取及其在食品安全检测中的应用
刘 嘉1,2,项锦欣3,刘洵妤1,3,赵国华1,3,*
(1.西南大学食品科学学院,重庆 400715;2.重庆市农产品加工技术重点实验室,重庆 400715;3.重庆理工大学化学与生物工程科学学院,重庆 400050)
支撑液膜作为一种新颖的萃取技术,以其选择性好、重现性好、节省有机溶剂等优点,逐渐成为萃取技术研究的主要方向。本文在介绍支撑液膜的工作原理、结构以及萃取影响因素的基础上,就支撑液膜萃取技术在食品中农药、化学污染物、重金属、食品添加剂及其他食品安全检测方面的应用和今后研究的重点进行综述,以期为食品安全控制技术研究提供有益的参考。
支撑液膜;萃取;检测;食品
液膜萃取技术是20世纪中后期发展起来的一种高效、快速、节能的新兴萃取技术。与固体膜分离技术相比,液膜萃取技术具有选择性强、传质面积大、分离效率高等优势。常见的液膜分离方式主要包括乳化液膜和支撑液膜。虽然乳化液膜的分离效果也非常优良,但因其操作复杂、液膜稳定性不高、分离后目标物质的回收困难等缺陷严重限制了应用。20世纪70年代,Bloch等[1]研究发展了支撑液膜,才使液膜萃取技术的应用得到空前的发展。与乳化液膜相比,支撑液膜拥有更好的液膜稳定性、选择性以及富集效果。近年来,在广泛深入研究的基础上,支撑液膜分离技术在环境分析、生物制品分离、医学以及食品安全分析中显示出了广阔的应用前景。本文在论述支撑液膜分离特点、技术原理和操作类型的基础上,详细介绍了支撑液膜技术在食品安全分析中的应用,以期为推动我国食品安全先进分析技术的研究提供参考。
1 支撑液膜萃取技术的原理
支撑液膜通常选用疏水性微孔膜作为支撑体,用特定有机溶剂浸泡时,微孔膜通过毛细孔力将有机溶剂束缚在其孔内,从而形成以固体载体支撑的有机溶剂液膜。从工作原理上看,支撑液膜分离技术实际上是由两个液液萃取构成的。一般将支撑液膜分离的被萃取液体相称为供体,用于接受被萃取物质的相称为受体。在分离过程中,样品液(供体)中的目标分离物质(B)首先通过供体与缚束在微孔中的液态有机溶剂形成的液液萃取过程转移至有机液膜中。接着,目标物质再通过微孔中的液态有机溶剂与受体形成的液液萃取转移至受体相中,从而实现目标物质的分离。图1为利用支撑液膜萃取酸性物质的原理图。
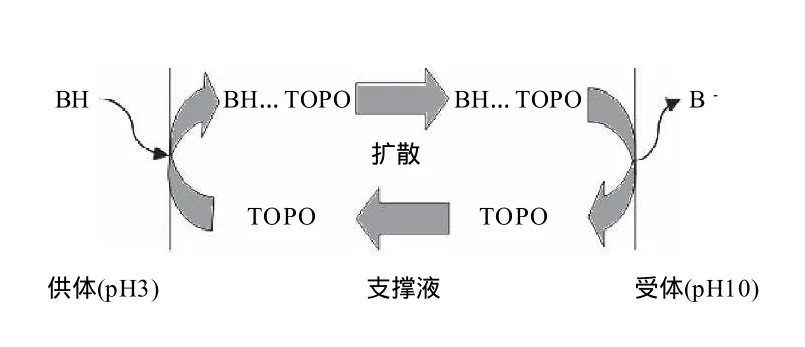
图2 含载体支撑液膜萃取示意图(以酸性物质为例)Fig.2 Schematic diagram of supported liquid membrane extraction
为实现目标物质从供体相转移至液膜,再从液膜中转入接受相,一般需要在形成有机液膜的溶剂中加入能特异性转移目标物质的载体。含载体的液膜萃取的过程实际上是一个反应萃取的过程(图2)。为实现分离,首先调节供体相的pH值至3使目标分离物质完全转变成分子状态(BH),处于分子状态的目标分离物质与溶解在有机液膜(如正己基醚)中的载体三辛基氧化磷 (TOPO)在液膜内侧形成氢键耦合物(BH…TOPO),进而被转移至液膜内。在浓度梯度的推动下氢键耦合物从液膜内侧扩散至液膜外侧。而在液膜外侧高pH值碱液的中和作用使耦合作用破坏,氢键耦合物分解,目标萃取物被转移至受体相,同时分子态目标萃取物(BH)被中和成离子态(B-)。由于离子态目标萃取物无法与TOPO形成耦合物,也就无法再回到液膜中,从而实现了目标物质的定向转移分离。解耦合后的TOPO扩散到液膜外侧再次利用。
2 支撑液膜的结构及萃取影响因素
2.1 支撑液膜的结构
支撑液膜的主体结构包括液膜及支撑体。液膜通常选取一些具有较高沸点及表面张力的有机溶剂,例如高级烷烃、苯、醚等。支撑体可分为两种构型,即平板膜和中空纤维膜。平板膜(图3)是通过供体相在支撑液膜的一侧不断地流动或者在供体相一端添加压力,从而加快了分析物在液膜中的传递。由于在两端添加了隔板,平板膜通常可以连续使用。中空纤维膜(图4)拥有相当高的填充比,并且单位料液体积的接触面积也大于平板膜,萃取效率更高。
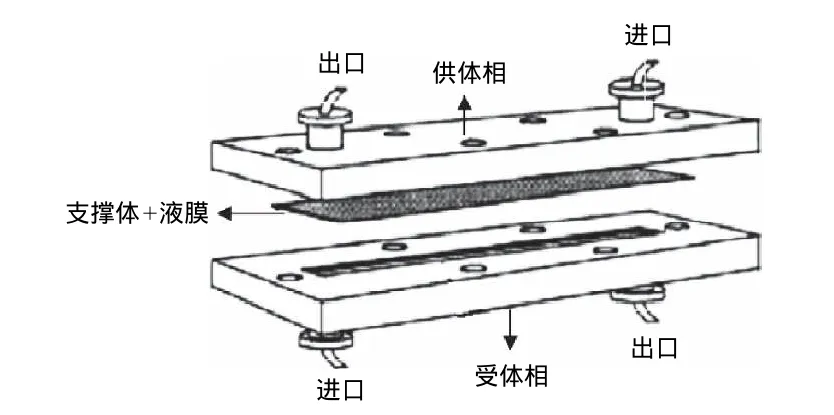
图3 平板式支撑液膜Fig.3 Flat-type supported liquid membrane
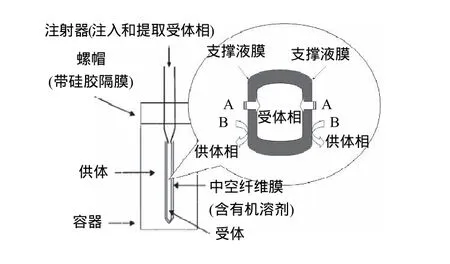
图4 中空纤维式支撑液膜Fig.4 Hollow-fiber-type supported liquid membrane
2.2 影响支撑液膜萃取的因素
2.2.1 温度
支撑液膜萃取的操作温度通常控制在室温或者稍高(25~35℃)。升高支撑液膜萃取的操作温度,可增加扩散系数,从而提高对目的物质的传质。液膜有机溶剂的沸点不高是限制操作温度的主要因素。有研究将离子液体应用到支撑液膜[2],一方面由于离子液体的沸点较高,可以通过提高操作温度以加快液膜对分析物的萃取速率;另一方面由于离子液体的黏度大,能有效地阻止液膜溢出,从而加强了支撑液膜的稳定性。
2.2.2 有机溶剂及载体
支撑液膜中被用于形成液膜的有机溶剂要求在供体相及受体相中的溶解性小,这样能较好地溶解分析物与载体,并且与支撑体有较好的兼容性[3]。有机溶剂的极性和对分析物的溶解能力对萃取效果的影响很大,可按一定比例混合使用多种有机溶剂[4]或选择使用合适的载体[5]。载体一方面可以大幅度提高萃取的选择性,另一方面能使萃取效率提高数十倍[6]。同时,为使液膜更为绿色、环保,有报道使用植物油作为液膜分离水中的苯酚[7]和染料[8]。
2.2.3 支撑体
常用于支撑液膜中支撑体的高分子材料包括聚四氟乙烯、聚丙烯、聚砜等低溶胀性及高孔隙率的疏水性材料,其厚度从几微米到几百微米不等。一般平板型支撑液膜的支撑体较中空纤维型更厚[9]。若需提高支撑体稳定性可选用较小孔径的材料,而要获得较好的通量则需选取较大孔径的材料。
2.2.4 其他因素
在萃取酸性物质时,供体相中的p H值应足够低,以确保目的物质质子化后能穿过液膜。接受相的pH值则根据实验具体要求进行调节,需要考虑对检测器的干扰且是否有利于载体释放分析物等因素。另外,对于平板模型的支撑体,适当调整其供体相流速能有效地增加料液与膜之间的接触时间,从而提高萃取效果[10]。
3 支撑液膜萃取技术在食品安全检测中的应用
作为一种优秀的萃取分离技术,支撑液膜萃取技术以其高富集能力与高选择性等特色已发展成为一种食品安全样品前处理技术,可用于农药、化学污染物、重金属、抗生素、食品添加剂等检测。
3.1 农药检测
研究表明利用支撑液膜进行样品前处理可用于包括氨基甲酸酯类、联啶类、硝基酚类、卤代苯氧类等农药的检测(表1)。Zhu等[11]利用支撑液膜富集分离了牛奶中卤代苯氧型除草剂,检测结果的相对平均偏差低于7.1%,检测在1~200ng/L范围内线性关系好,被检测物的富集倍数可达700。Piriyapittaya等[12]使用溶有载体(季胺化合物-336)的正己基醚作为膜液萃取纯水中草甘膦和胺甲基磷酸。目标物的萃取效果好,其富集倍数可达853。Msagati等[13]发现支撑液膜对苯并咪唑进行富集分离的回收率高于固相微萃取。此外,支撑液膜还可用于对红酒中果汁[10]、杀虫剂[14]、和水[15]中三嗪除草剂的富集分离。
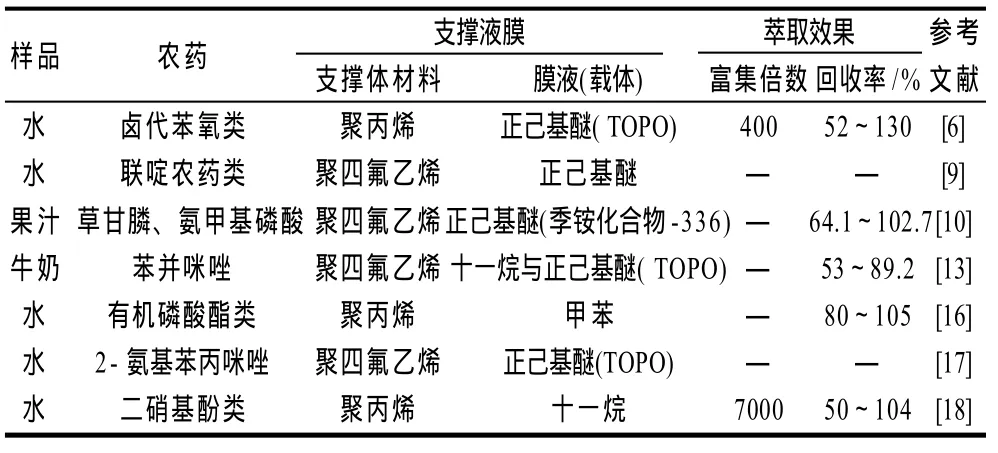
表1 支撑液膜技术在农药检测上的应用Table 1 Application of supported liquid membrane in the inspection of pesticides
3.2 化学污染物检测
支撑液膜可以用于水体中氯化消毒副产物(卤乙酸)、多环芳烃类、氯酚类等污染物的检测(表2)。Kou等[2]使用离子液体作为膜液富集分离水中卤乙酸类污染物,富集倍数在300~3000之间,且萃取率高于50%。Liu等[4]以三氯甲烷作为膜液,实现了对水体中5种氯酚类化合物的分离。Peng等[19]发现以离子液体作为膜液时,重复性更好。
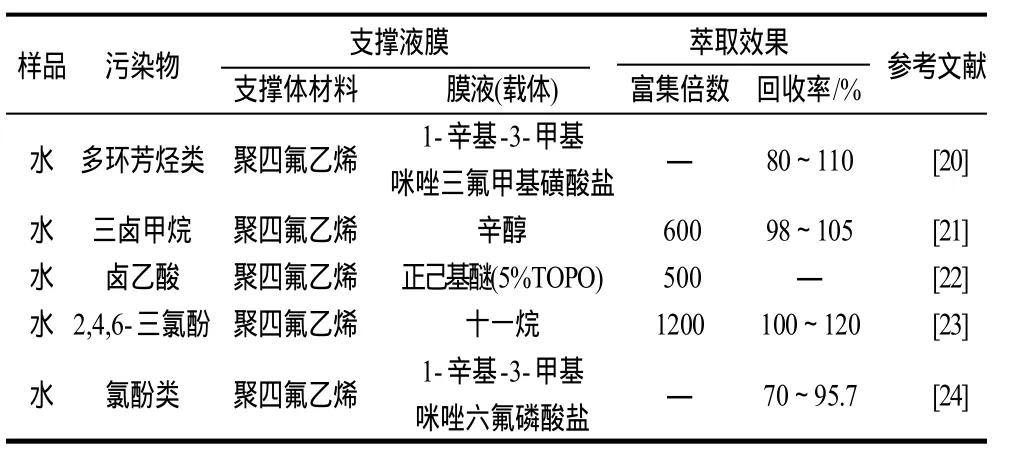
表2 支撑液膜技术在化学污染物检测上的应用Table 2 Application of supported liquid membrane in the inspection of chemical contaminants
3.3 重金属检测
可利用支撑液膜技术对水样中铜、铬、铅等重金属进行富集分离(表3)。Parthasarathy等[25]在对微量铜、铅、铬的富集分离中,以甲苯和苯基己烷的混合溶液作为膜液,富集倍数可达1000。Peng等[26]研究了以双硫腙作为萃取载体富集分离铬,其富集倍数可达387。Cukrowska等[27]以四乙基硼酸钠为载体对氯化三苯锡、氯化三叔丁基锡、三氯化叔丁基锡、二氯化二叔丁基锡等有机锡进行富集分离,富集倍数可达1000~4000。
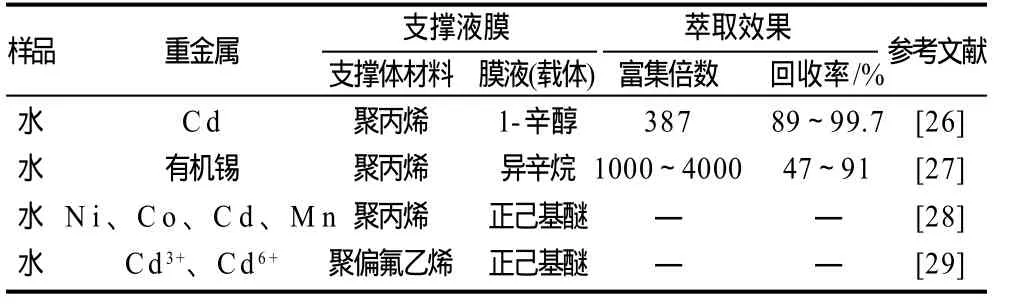
表3 支撑液膜技术在重金属检测上的应用Table 3 Application of supported liquid membrane in the inspection of heavy metals
3.4 抗生素检测
抗生素是当前动物性食品重点的污染物,在分析监控磺胺类、乳胺类等时可用支撑液膜对样品进行分离富集(表4)。Msagati等[5]利用支撑液膜富集分离了水、牛奶和牛肝肾等多种样品中的磺胺类药物。同时,他们还发现利用支撑液膜萃取分离β-乳胺类抗生素的萃取效率比固相微萃取好,可使目标物质的检测限大幅度降低。Raich等[30]应用支撑液膜富集分离双氢除虫菌素,其萃取效率与固相微萃取相当,但萃取时间更短、有机溶剂使用更少。
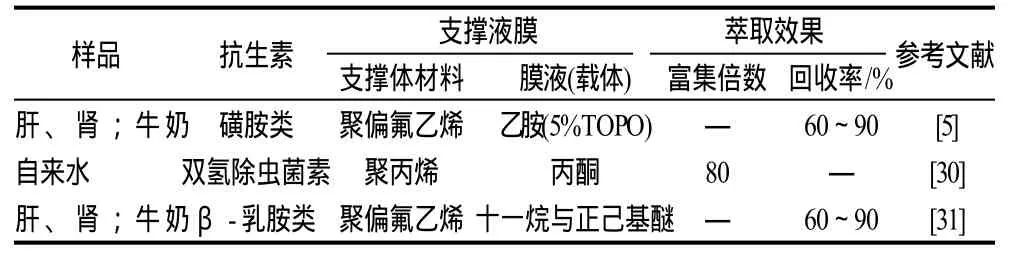
表4 支撑液膜技术在抗生素检测上的应用Table 4 Application of supported liquid membrane in the inspection of antibiotics
3.5 食品添加剂检测
Luque-Perez等[32]将支撑液膜应用于咖啡因的富集,并设计出一套流动进样系统,实现了对目标物的在线检测。Zougagh等[33]使用十一烷与己醚作为膜液萃取分离咖啡和茶叶中的咖啡因,并把压电晶体、分子印迹聚合物等技术应用于检测。结果表明,目标物的回收率为90%~109%,分子印迹技术增强了分离的选择性,而压电晶体提高了方法的检测限。Avila等[34]还利用支撑液膜对食品中香草醛进行了富集。Luque等[35]将支撑液膜与安培计结合,分别对糖果、巧克力涂层饼干、巧克力3种食品中的香草醛进行检测,富集倍数可达700。3.6 其他方面检测
Liu等[36]利用支撑液膜富集分离了自来水、河水、城市污水样品中的双酚A类激素,在萃取30min后,3种样品的回收率在76%~116%之间。Almeda等[37]使用辛醇作为膜液富集分离了红酒中赭曲霉毒素并结合毛细管电泳进行检测,提高了测定的灵敏度,并且测定的相对标准偏差在5%以内。
4 结 语
支持液膜萃取是一种极具发展潜力的环境友好型萃取技术。与传统的溶剂萃取相比,它具有传质速度快、富集倍数高、选择性强、节省样品、节省有机溶剂等优点。该技术目前被广泛研究应用于环境分析、生化分析、分子相互作用分析、食品安全分析等领域。在食品安全领域中,它可用于食品中农药、化学污染物、重金属、食品添加剂的检测。但目前支撑液膜的应用主要集中在环境分析中,在食品分析中的研究仍相对偏少,还有很大发展空间。难于自动化和萃取的稳定性是限制当前支持液膜萃取的主要技术因素。便携式小型支撑液膜萃取装置、液膜稳定技术以及支撑液膜与现代分析仪器偶联技术的深度开发对支撑液膜在工业分析中大面积应用至关重要。
[1] BLOCH R, FROMMER M A. The mechanism for formation of skinned membranes I. Structure and properties of membranes cast from binary solutions[J]. Desalination, 1970, 7(2): 259-264.
[2] KOU D, WANG X Y, MITRA S. Supported liquid membrane microextraction with high-performance liquid chromatography-UV detection for monitoring trace haloacetic acids in water[J]. Journal of Chromatography A, 2004, 1055(1/2): 63-69.
[3] KHROLENKO M V, WIECZOREK P P. Determination of glyphosate and its metabolite aminomethylphosphonic acid in fruit juices using supported-liquid membrane preconcentration method with high-performance liquid chromatography and UV detection after derivatization withp-toluenesulphonyl chloride[J]. Journal of Chromatography A, 2005, 1093(1/2): 111-117.
[4] LIU Jingfu, XIA Liang, CHI Yuguang, et al. High performance liquid chromatography determination of chlorophenols in water samples after preconcentration by continuous flow liquid membrane extraction on-line coupled with a precolumn[J]. Analytica Chimica Acta, 2003, 487(2): 129-135.
[5] MSAGATI T A M, NINDI M M. Multiresidue determination of sulfonamides in a variety of biological matrices by supported liquid membrane with high pressure liquid chromatography-electrospray mass spectrometry detection[J]. Talanta, 2004, 64(1): 87-100.
[6] LIU Jingfu, TORANG L, JONSSON J A. Passive extraction and cleanup of phenoxy acid herbicides in samples from a groundwater plume using hollow fiber supported liquid membranes[J]. Journal of Chromatography A, 2007, 1160(5): 56-63.
[7] VENKATESWARAN P, PALANIVELU K. Recovery of phenol from aqueous solution by supported liquid membrane using vegetable oils as liquid membrane[J]. Journal of Hazardous Materials B, 2006, 131(1/3): 146-152.
[8] MUTHURAMAN G, PALANIVELU K. Transport of textile dye in vegetable oils based supported liquid membrane[J]. Dyes and Pigments, 2006, 70(2): 99-104.
[9] MULUGETA M, MEGERSA N. Carrier-mediated extraction of bipyridilium herbicides across the hydrophobic liquid membrane[J]. Talanta, 2004, 64(1): 101-108.
[10] KHROLENKO M, DZYGIEL P, WIECZOREK P. Combination of supported liquid membrane and solid-phase extraction for sample pretreatment of triazine herbicides in juice prior to capillary electrophoresis determination[J]. Journal of Chromatography A, 2002, 975(1): 219-227.
[11] ZHU Lingyan, EE K H, ZHAO Limian, et al. Analysis of phenoxy herbicides in bovine milk by means of liquid-liquid-liquid microextraction with a hollow-fiber membrane[J]. Journal of Chromatography A, 2002, 963(1/2): 335-343.
[12] PIRIYAPITTAYA M, JAYANTA S, LEEPIPATPIBOON N, et al. Micro-scale membrane extraction of glyphosate and aminomethylphosphonic acid in water followed by high-performance liquid chromatography and post-column derivatization with fluorescence detector[J]. Journal of Chromatography A, 2008, 1189(1/2): 483-492.
[13] MSAGATI T A M, NINDI M M. Comparative study of sample preparation methods; supported liquid membrane and solid phase extraction in the determination of benzimidazole anthelmintics in biological matrices by liquid chromatography-electrospray-mass spectrometry[J]. Talanta, 2006, 69(1): 243-250.
[14] HYOTYLAINEN T, LUTHJE K, RAUTIAINEN R M, et al. Determination of pesticides in red wines with on-line coupled microporous membrane liquid-liquid extraction-gas chromatography[J]. Journal of Chromatography A, 2004, 1056(1/2): 267-271.
[15] BJARNI B, LUKE C, PATRIK O, et al. Enzyme flow immunoassay using a Protein G column for the screening of triazine herbicides in surface and waste water[J]. Analytica Chimica Acta, 2001, 426(2): 197-207.
[16] LAMBROPOULOU D A, ALBANIS T A. Application of hollow fiber liquid phase microextraction for the determination of insecticides in water[J]. Journal of Chromatography A, 2005, 1072(1): 55-61.
[17] SANDAHL M, MATHIASSON L, JONSSON J A. Determination of thiophanate-methyl and its metabolites at trace level in spiked natural water using the supported liquid membrane extraction and the microporous membrane liquid-liquid extraction techniques combined on-line with high-performance liquid chromatography[J]. Journal of Chromatography A, 2000, 893(1):123-131.
[18] BERHANU T, LIU J F, JONSSON J A K, et al. Determination of trace levels of dinitrophenolic compounds inenvironmental water samples using hollow fiber supported liquidmembrane extraction and high performance liquid chromatography[J]. Journal of Chromatography A, 2006, 1103(1): 1-8.
[19] PENG Jinfeng, LIU Jingfu, HU Xialin, et al. Direct determination of chlorophenols in environmental water samples by hollow fiber supported ionic liquid membrane extraction coupled with high-performance liquid chromatography[J]. Journal of Chromatography A, 2007, 1139 (2): 165-170.
[20] HSIEH Y N, HUANG P C, KUEI C H. Nafion membrane-supported ionic liquid-solid phase microextraction for analyzing ultra trace PAHs in water samples[J]. Analytica Chimica Acta, 2006, 557(2): 321-328.
[21] VORA-ADISAK N, VARANUSUPAKUL P. A simple supported liquid hollow fiber membrane microextraction for sample preparation of trihalomethanes in water samples[J]. Journal of Chromatography A, 2006, 1121(2): 236-241.
[22] WANG Xiaoyan, KOU Dawen, MITRA S. Continuous on-line monitoring of haloacetic acids via membrane extraction[J]. Journal of Chromatography A, 2005, 1089(2): 39-44.
[23] TUDORACHE M, EMNEUS J. Selective immuno-supported liquid membrane (ISLM) extraction,enrichment and analysis of 2,4,6-trichlorophenol[J]. Journal of Membrane Science, 2005, 256(1/2): 143-149.
[24] ALMEDA S, NOZAL L, VALCARCEL M. Direct determination of chlorophenols present in liquid samples by using a supported liquid membrane coupled in-line with capillary electrophoresis equipment[J]. Analytica Chimica Acta, 2007, 587(1): 97-103.
[25] PARTHASARATHY N, PELLETIER M, BUFFLE J. Hollow fiber based supported liquid membrane: a novel analytical system for trace metal analysis[J]. Analytica Chimica Acta, 1997, 350(1/2): 183-195.
[26] PENG Jinfeng, LIU Rui, LIU Jingfu, et al. Ultrasensitive determination of cadmium in seawater by hollow fiber supported liquid membrane extraction coupled with graphite furnace atomic absorption spectrometry [J]. Spectrochimica Acta Part B, 2007, 62(5): 499-503.
[27] CUKROWSKA E, CHIMUKA L, NSENGIMANA H, et al. Application of supported liquid membrane probe for extraction and preconcentration of organotin compounds from environmental water samples[J]. Analytica Chimica Acta, 2004, 523(1): 141-147.
[28] NDUNGU K, DJANE N K, MATHIASSON L. Determination of trace metal ions by ion-pair chromatography after enrichment using supported liquid membrane[J]. Journal of Chromatography A, 1998, 826(1): 103-108.
[29] DJANE N K, NDUNGU K, MATHIASSON L, et al. Chromium speciation in natural waters using serially connected supported liquid membranes [J]. Talanta, 1999, 48(5): 1121-1132.
[30] RAICH M J, KROGH K A, GRANADOS M, et al. Determination of ivermectin and transformation products in environmental waters using hollow fibre-supported liquid membrane extraction and liquid chromatography-mass spectrometry/mass spectrometry[J]. Journal of Chromatography A, 2008, 1187(1/2): 275-280.
[31] MSAGATI T A M, NINDI M M. Determination ofβ-lactam residues in foodstuffs of animal origin using supported liquid membrane extraction and liquid chromatography-mass spectrometry[J]. Food Chemistry, 2007, 100(2): 836-844.
[32] LUQUE-PEREZ E, RIOS A, VALCARCEL M, et al. Spectrophotometric flow injection determination of caffeine in solid and slurry coffee and tea samples using supported liquid membranes[J]. Laboratory Automation and Information Management, 1999, 34(2): 131-142.
[33] ZOUGAGH M, RIOS A, VALCARCEL M. Automatic selective determination of caffeine in coffee and tea samples by using a supported liquid membrane-modified piezoelectric flow sensor with molecularly imprinted polymer[J]. Analytica Chimica Acta, 2005, 539(1/2): 117-124.
[34] AVILA M, ZOUGAGH M, RIOS A, et al. Supported liquid membranemodified piezoelectric flow sensor with molecularly imprinted polymer for the determination of vanillin in food samples[J]. Talanta, 2007, 72 (4): 1362-1369.
[35] LUQUE M, LUQUE-PEREZ E, VALCARCEL M, et al. Supported liquid membranes for the determination of vanillin in food samples with amperometric detection[J]. Analytica Chimica Acta, 2000, 410(1/2): 127-134.
[36] LIU Jingfu, XIA Liang, JIANG Guibin, et al. Evaluation of an on-line coupled continuous flow liquid membrane extraction and precolumn system as trace enrichment technique by liquid chromatographic determination of bisphenol A[J]. Talanta, 2003, 60(6): 1155-1161.
[37] ALMEDA S, ARCE L, VALCARCEL M. Combined use of supported liquid membrane and solid-phase extraction to enhance selectivity and sensitivity in capillary electrophoresis for the determination of ochratoxin A in wine[J]. Electrophoresis, 2008, 29(7): 1573-1581.
Supported Liquid Membrane Extraction and Its Application in Food Safety Inspection
LIU Jia1,2,XIANG Jin-xin3,LIU Xun-yu1,3,ZHAO Guo-hua1,3,*
(1. College of Food Science, Southwest University, Chongqing 400715, China;2. Chongqing Key Laboratory of Agricultural Product Processing, Chongqing 400715, China;3. College of Chemical and Bioengineering, Chongqing Technology University, Chongqing 400050, China)
Supported liquid membrane extraction, a novel extract technique with advantages of economic organic solvents, high selectivity and good reproducibility has been attracted extensive attention. In this paper, working principle, structure and factors of this extraction technique were introduced and its applications in the detection of pesticides, chemical pollutants, heavy metals, and other toxic substances in food was discussed, which will provide valuable references for food safety control and inspection.
supported liquid membrane;extraction;inspection;food
TS201.21
A
1002-6630(2010)13-0321-05
2009-12-03
重庆市自然科学基金项目(CSTC 2007BB1393)
刘嘉(1985—),男,硕士研究生,研究方向为食品化学。E-mail:mcgrady456@163.com
*通信作者:赵国华(1971—),男,教授,博士,研究方向为食品化学。E-mail:zhaoguohua1971@163.com
