脂滴
——细胞脂类代谢的细胞器
2010-09-13张淑妍杜雅兰汪洋刘平生
张淑妍,杜雅兰,2,汪洋,刘平生
1.中国科学院生物物理研究所,北京100101;
2.贵州大学生命科学学院,贵阳550025
脂滴
——细胞脂类代谢的细胞器
张淑妍1,杜雅兰1,2,汪洋1,刘平生1
1.中国科学院生物物理研究所,北京100101;
2.贵州大学生命科学学院,贵阳550025
脂滴是细胞内中性脂贮存的主要场所,由极性单磷脂层包裹疏水核心组成。近年来的蛋白质组学研究表明,脂滴表面还存在着许多功能蛋白,进一步揭示了脂滴可能参与细胞内物质的代谢和转运,以及细胞信号传导等过程,是一个活动旺盛的多功能细胞器。实验结果还证明,脂滴不但是甘油三酯贮存和分解、花生四烯酸代谢和前列腺素合成的主要场所,脂滴还具有合成甘油三酯和磷酯的功能。由此可见,脂滴可能是细胞内参与脂类合成代谢的细胞器。
脂滴;蛋白质组学;酶;脂类代谢;细胞器
0 引言
脂滴是细胞内中性脂(neutral lipids)的主要贮存场所,广泛存在于细菌、酵母、植物、昆虫以及动物细胞中[1,2]。脂滴的大小差别很大,直径从40 nm至100 μm不等[3]。脂滴由磷脂单分子层及中性脂构成的疏水核心构成,并且表面分布有很多蛋白。最早关于脂滴的描述出现在1674年,van Leeuwenhoeck在牛奶中发现了脂肪滴。19世纪末,Altmann[4]和Wilson[5]也观察到了脂滴,并命名为脂质体(liposome)。在对脂滴的不断研究中,又陆续出现了很多名字,如lipid droplet、lipid body、fat body、fat droplet,以及最近的adiposome[6]。目前来看,大部分科研工作者仍习惯称之为lipid droplet[7]。
长期以来,脂滴一直被认为是一种类似于糖原的颗粒,只是用来贮存能量,当细胞需要能量时,用来供给能量,是一个“惰性”的细胞内含物,因而脂滴在很长一段时间内并未受到人们的重视。1991年,Greenberg等人[8]发现了第一个与脂肪细胞脂滴相关的蛋白perilipin。随后,Jiang等人[9]于1992年克隆了脂滴的主要蛋白ADRP。加上后来在脂滴上发现的Tip47[10],Miura等人[11]于2002年定义了PAT(Perilipin,ADRP,Tip47)家族蛋白。近来PAT家族又增加了S3-12[12]和OXPAT/PAT1[13]两个蛋白。2004年,Liu[6]建立了脂滴的纯化方法,并与同事用蛋白质组学方法发现动物细胞脂滴不但含有PAT家族蛋白,还带有甘油三酯水解酶(adipose triglyceride lipase,ATGL)和与脂肪合成及胆固醇合成相关的许多酶类,以及与膜转运相关的蛋白,如小G蛋白Rabs。2007年,Bartz等人[14]首次完成了脂滴的脂质组学分析并发现脂滴中存在另一种中性脂:醚脂。在这些工作,以及近年来越来越多的脂滴蛋白质组学研究的推动下,有关脂滴的研究得到迅速发展。研究表明,脂滴并非细胞内一个简单的能量贮存器,而是一个复杂、活动旺盛、动态变化的多功能细胞器。脂滴能够沿着细胞骨架运动,并与其它细胞器相互作用,可能在脂类代谢与存储、膜转运、蛋白降解,以及信号传导过程中起着重要的作用[2,15,16](图1)。另外,研究还表明,多种代谢性疾病,如肥胖、脂肪肝、心血管疾病[17]及糖尿病[18,19]、中性脂贮存性疾病[19]和Niemann Pick C疾病[20],往往都伴随着脂质贮存的异常。因此,关于脂滴的生物学研究日益受到人们的重视。虽然脂滴在细胞中可能行使许多功能,限于篇幅,本文仅着重讨论脂滴在脂类代谢中所起的作用。

图1 脂滴的结构与功能脂滴由极性单分子层(如磷脂和胆固醇)、非极性核心(如胆固醇酯和甘油三酯),以及表面的许多功能蛋白构成。脂滴表面蛋白研究表明,脂滴可能参与细胞的脂质合成与代谢、物质的运输及贮存,以及细胞信号传导过程Fig.1StructureandfunctionsoflipiddropletsAlipiddropletconsistsofaphospholipid monolayer that surrounds a hydrophobic core.Recent study reveals that lipid droplets may be a dynamic organelle in metabolism,transport and signal transduction
1 脂滴中含有丰富的与脂类代谢相关的酶
近年来,人们已经从9种不同的细胞及组织中分离纯化出脂滴,进行了15项脂滴蛋白组学的研究[6,21~34]。到目前为止,已发现的哺乳动物脂滴蛋白有120多种[33]。分析发现,有很多蛋白是与甘油三酯、磷脂和胆固醇的合成、修饰、降解等脂类代谢有关的酶。例如,Liu等人[6]对中国仓鼠卵巢细胞CHO K2的脂滴进行质谱学和免疫印迹分析,鉴定出将近40种蛋白,其中约35%是参与脂类代谢的酶。Umlauf等人[23]用MALDI-TOF MS方法在人表皮鳞状细胞癌A431细胞株中鉴定出了33个脂滴蛋白,其中有7个是脂代谢相关的酶。Fujimoto等人[24]用人肝癌细胞系HuH7,利用nano LC-MS/MS方法,对其脂滴蛋白进行分析,鉴定出17个主要的蛋白,其中5个是与脂类代谢有关的酶。这些结果表明细胞内的脂滴可能是参与脂代谢的细胞器之一。
2 脂滴在脂类代谢中的作用
2.1 脂滴参与甘油三酯的合成
细胞中甘油三酯(triacylglycerol,TAG)的合成大部分从磷酸甘油(glycerolphosphate)开始,然后,脂肪酸单体依次加入到甘油的骨架上。研究表明,脂滴中存在着甘油三酯合成通路中的多种酶,如脂酰CoA合成酶(acyl-CoA synthetase long-chain family,ACSL)和酰基转移酶,如二酯酰甘油酰基转移酶(diacylglycerol acyltransferase,DGAT)等。
Kuerschner等人[35]发现,COS7细胞及3T3-L1脂肪细胞的脂滴中不仅存在着甘油三酯,还存在着其合成前体甘油二酯(diacylglycerol,DAG),而且脂滴中还存在着催化这一反应的酶DGAT2。在分离纯化的脂滴中,加入棕榈酰CoA(palmiltoyl-CoA)和用同位素标记的DAG,则体系中将有放射性标记的TAG生成[36]。
由蛋白组学的结果可以看出,ACSL广泛存在于不同种类的哺乳动物细胞脂滴中。ACSL是一个蛋白家族,该家族以长链脂肪酸、ATP和CoA为底物,催化脂酰CoA的生成。脂酰CoA是合成其它脂类(如TAG和胆固醇酯)的重要底物。哺乳动物中有5个ACSL的同工酶,ACSL1及ACSL3~ACSL6。Fujimoto等在人肝癌细胞HuH7中鉴定出ACSL3是脂滴相关蛋白,而且他们发现,将纯化的脂滴和14C标记的油酸(oleic acid)或者棕榈酸(palmitic acid)一起孵育,将会有放射性的脂酰CoA生成,表明脂滴具有ACSL活性;脂滴与14C标记的油酰-CoA(oleoyl-CoA)一起孵育,同时加入DAG或胆固醇,将会有放射性的TAG和胆固醇酯生成,表明脂滴能够部分参与中性脂的合成,并且具有DGAT及脂酰CoA:胆固醇酰基转移酶(acyl-CoA:cholesterol acyltransferase,ACAT)的活性。而且,在不加入DAG和胆固醇的情况下,脂滴仍然能够合成TAG和胆固醇酯,表明脂滴自身可能提供脂代谢的部分中间产物[37]。因此,脂滴至少在一定程度上参与了细胞内TAG的合成。
2.2 脂滴在降解甘油三酯中的作用
激素敏感性脂肪酶(hormone-sensitive lipase,HSL)是脂滴上首先被发现的催化中性脂水解的脂肪酶[38]。第二个被发现的酶被称为脂肪甘油三酯脂肪酶(adipose triglyceride lipase,ATGL)、desnutrin或Ca2+依赖的磷脂酶A2(phospholipase A2),这种酶能够将TAG水解为DAG[39]。
HSL在正常情况下主要存在于胞浆中,在刺激脂肪水解的条件下,几乎完全定位于脂滴上[40,41]。这种定位是由蛋白磷酸激酶A(protein kinase A,PKA)介导的perilipin以及HSL的磷酸化来调控的。
ATGL是在脂肪细胞中发现的[42]。研究表明它也存在于其它组织中,而且定位于脂滴表面[6]。虽然通过磷酸化蛋白质组学研究,已发现ATGL是一个磷酸化蛋白[33],但其调控ATGL活性的分子机理至今仍不清楚。Lass等人[43]的研究工作发现定位于脂滴上的一种蛋白——CGI-58[6]可以激活ATGL的酶活性。CGI-58是α/β-水解酶折叠(α/β-hydrolase fold enzyme)家族蛋白的一员。将CGI-58缺失,能够引起遗传性的中性脂贮存紊乱,原因在于大量的甘油三酯累积在细胞中[44]。表明脂滴上的CGI-58极有可能参与甘油三酯的降解。
2.3 脂滴参与胆固醇代谢
酵母脂滴的蛋白质组学研究表明,脂滴上存在麦角固醇(ergosterol)合成过程中所需要的羊毛固醇合酶(lanosterol synthase),如Erg6和Erg7[21]。Won-Ki Huh等人[45]用GFP/RFP双荧光蛋白将Erg6定位在脂滴上。由蛋白组学分析的结果可以看出,该酶在许多哺乳动物细胞的脂滴中都有分布,表明脂滴可能是合成羊毛固醇的场所。在酵母中,该酶几乎只存在于脂滴[46]。有意思的现象是,只有几种胆固醇合成酶定位于脂滴上,而且从胆固醇合成的途径上看,它们是不连续的。同样,脂滴有可能参与胆固醇类的分解代谢。例如,巨噬细胞以及乳腺上皮细胞中的脂滴富含胆固醇酯,其脂滴中往往含有胆固醇酯酶(cholesterol esterase)[22,47]。
2.4 脂滴参与花生四烯酸代谢
花生四烯酸(arachidonic acid,AA)是一种重要的多不饱和脂肪酸,是前列腺素类物质以及白三烯(leukotriene)类物质的前体。研究表明,白细胞脂滴上含有AA的胞浆磷脂酶A2(cytosolic phospholipase,cPLA2)、合成白三烯的5-脂氧合酶(5-lipoxygenase,5-LO)、生成前列腺素的环氧化酶(cyclooxygenase,COX)和前列腺素H合成酶(prostaglandin H synthase,PGHS),以及白三烯C4合成酶(leukotriene C4 synthase,LTC4S)[48~51]。电子显微放射自显影结果显示,外源标记的AA将会进入嗜酸细胞、中性细胞、肥大细胞、巨噬细胞和上皮细胞的脂滴中。将脂滴纯化出来之后,可以发现脂滴是AA的贮存场所[52,53]。白细胞中脂滴增多,往往伴随着前列腺素E2(prostaglandin E2,PGE2)和类二十烷酸(eicosanoid)的增加[49]。另外,免疫定位表明脂滴是从头合成LTC4和PGE2的场所[48]。即使不是在白细胞中,脂滴在类二十烷酸合成中可能也起作用。例如,在胎儿上皮细胞和成纤维细胞的脂滴中,同样存在着cPLA2和PGHS[54]。用油酸或AA等不饱和脂肪酸诱导大鼠肠上皮细胞,能够使脂滴迅速聚集,并且新合成的脂滴中含有cPLA2α[55]。在体外,将14C标记的1-palmitoyl-2-arachidonyl phosphatidylcholine与纯化的脂滴孵育,可以检测到放射性AA的生成,表明脂滴具有cPLA2酶活性[56]。由此可见,脂滴中存在类二十烷酸合成所需的底物及全部的酶,脂滴是类二十烷酸合成的场所[49,53]。
我们将上面介绍的脂滴中含有的与脂类代谢相关的酶总结于表1中。
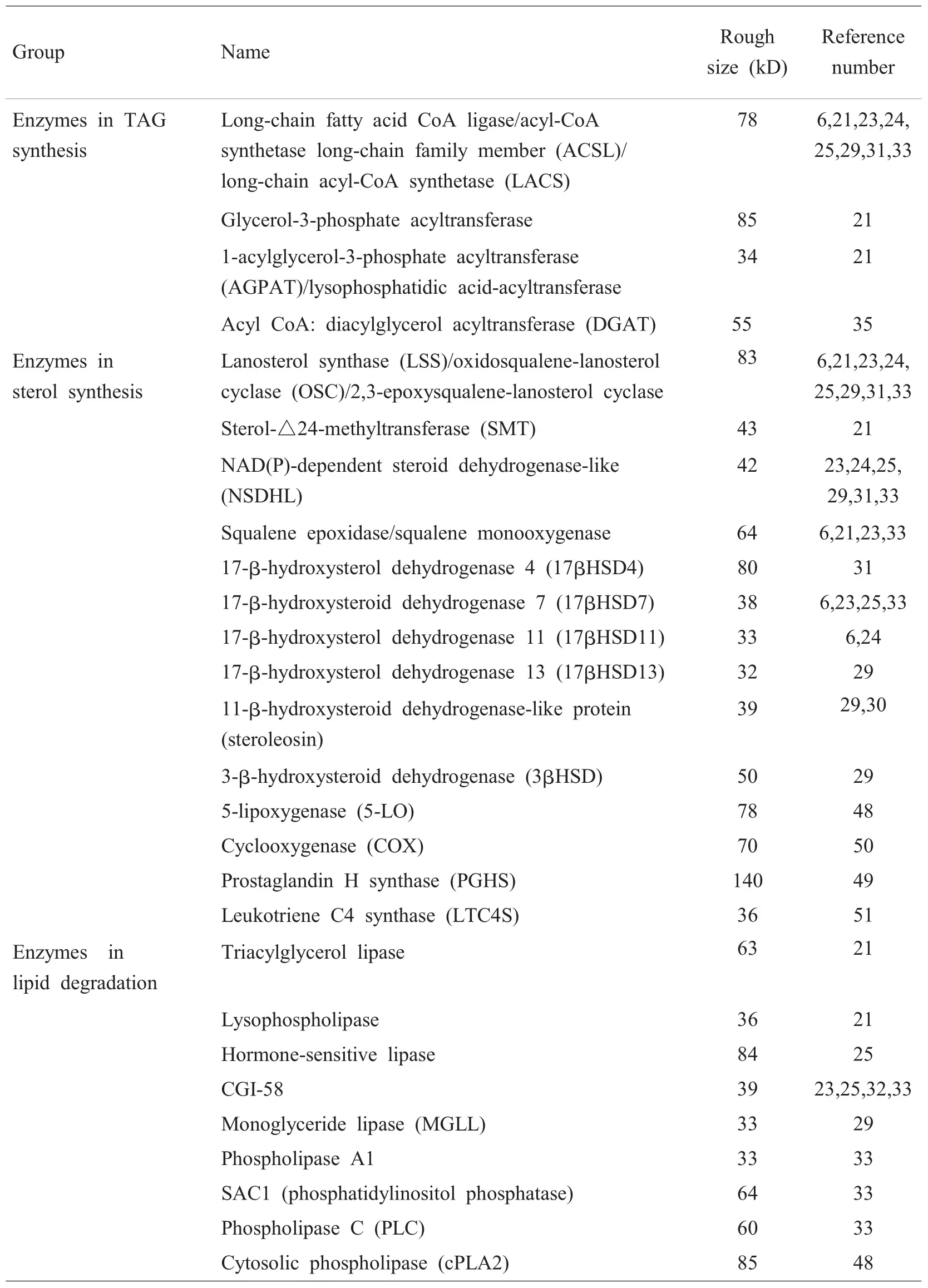
表1 脂滴中参与脂质代谢的酶Table 1Lipid droplet-associated lipid metabolic enzymes
3 脂滴中的其它脂类代谢相关蛋白
脂滴表面存在着合成磷脂的关键酶CCT(三磷酸胞苷:磷酸胆碱胞苷酰基转移酶,CTP:phosphocholine cytidylyltransferase)[57]。2000年,Wu等人[58]在小鼠乳腺上皮细胞的脂滴鉴定出了丙酮酸羧化酶,该酶在Ca2+浓度升高时,催化合成草酰乙酸,该产物将进入生脂途径。
此外,除存在于脂滴上的与脂质代谢相关的酶外,在脂滴上还存在着脂质代谢过程中所需要的其它因子。如在AA代谢过程中的蛋白S100A9,负责将不饱和脂肪酸转运到膜上[59]。在肝细胞Hep39的脂滴中,发现了固醇携带蛋白(sterol carrier protein)和脂肪酸结合蛋白,这些蛋白在脂代谢过程中起着重要的作用[31]。另外,在脂滴上还发现有能够结合胆固醇和长链脂肪酸的caveolin-1和caveolin-2[6,60~62]。Caveolin是caveolae的主要结构蛋白,同时具有与脂质和其它蛋白相互作用的功能[63]。这意味着这两种细胞器之间有着某种相互作用。
4 结论及展望
从脂滴中所含的蛋白特性来看,脂滴包含了合成、贮存、利用和降解多种脂类的酶,并且在脂类代谢(包括甘油三酯合成及降解、固醇类物质的代谢)中起着重要的作用,是脂类代谢活跃的细胞器。而且脂滴可能在脂类和能量代谢中处于核心地位,是调控细胞内脂类平衡的核心点[64,65]。
但是,不难看出,脂滴中仅仅包含着脂类代谢中的一部分酶,每一条代谢通路在脂滴中仅仅存在着几个酶,而且它们催化的往往是不连续的几步反应。因此,脂滴中脂类代谢的途径并不完整。例如,对酵母中带有GFP标签的蛋白进行分析,仅在脂滴中发现了麦角固醇代谢途径中的蛋白Erg1、Erg6和Erg7[33]。很有可能,只有在特定条件下,代谢过程中所需要的其它酶才会聚集到脂滴表面[33]。
正如其它生物学事件一样,例如代谢、大分子的生物合成、能量转化、蛋白运动、信号传递等,都需要细胞器之间的相互作用。脂滴中脂类代谢需要与其它细胞器相互作用。电镜结果表明,脂滴与线粒体、内质网(endoplasmic reticulum,ER),以及过氧化物酶体(peroxisome),在空间上很接近[66~68]。脂滴是脂肪酸丰富的来源,而脂肪酸作为线粒体和过氧化物酶体的氧化底物,脂滴与其相互作用就会使这一过程的效率增加。Athenstaedt等人[21]发现,在特定条件下,Erg1会从脂滴转移到ER上。事实上,部分线粒体蛋白在脂滴的蛋白质组中也会出现[25,28]。
可见,脂滴能够与其它细胞器很活跃地交换产物,因此,脂滴可能扮演着双重角色,代谢活跃的场所或者是细胞内物质运输时停靠的码头[27]。
在过去的几年中,关于脂滴的细胞生物学研究取得了很大的进展,脂滴作为一个重要的、活跃的细胞器,其重要的生物学功能正为人们所逐渐认识。但是,涉及到脂滴的很多关键内容还不清楚。比如,脂滴到底是如何形成的?蛋白是如何定位于脂滴上的?脂滴的融合和裂解是如何实现的?脂滴与其它细胞器之间的相互作用是如何调控的?对于这些问题的回答,可能会揭示出关于脂滴、细胞能量代谢等重大生物学问题的答案[65]。
1.Murphy DJ.The biogenesis and functions of lipid bodies in animals,plants and microorganisms.Prog Lipid Res,2001, 40(5):325~438
2.Zehmer JK,Huang Y,Peng G,Pu J,Anderson RG,Liu P.A role for lipid droplets in inter-membrane lipid traffic. Proteomics,2009,9(4):914~921
3.Stobart AK,Stymne S,Hglund S.Safflower microsomes catalyse oil accumulationin vitro:a model system.Planta, 1986,169:33~37
4.Altmann R.Die Elementarorganisem und ihre Beziehungen zu den Zellen.Leipzig:Veit,1890
5.Wilson E.The cell in development and inheritance.New York:Macmillan,1896
6.Liu P,Ying Y,Zhao Y,Mundy DI,Zhu M,Anderson RG. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic.J Biol Chem,2004,279(5):3787~3792
7.Farese RV Jr,Walther TC.Lipid droplets finally get a little R-E-S-P-E-C-T.Cell,2009,139(5):855~860
8.Greenberg AS,Egan JJ,Wek SA,Garty NB,Blanchette-MackieEJ,LondosC.Perilipin,amajorhormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets.J Biol Chem,1991, 266(17):11341~11346
9.Jiang HP,Serrero G.Isolation and characterization of a full-lengthcDNAcodingforanadipose differentiation-related protein.Proc Natl Acad Sci USA, 1992,89(17):7856~7860
10.Wolins NE,Rubin B,Brasaemle DL.TIP47 associates with lipid droplets.J Biol Chem,2001,276(7):5101~5108
11.Miura S,Gan JW,Brzostowski J,Parisi MJ,Schultz CJ, Londos C,Oliver B,Kimmel AR.Functional conservation for lipid storage droplet association among perilipin,ADRP, and TIP47(PAT)-related proteins in mammals,Drosophila, andDictyostelium.JBiolChem,2002,277(35): 32253~32257
12.Wolins NE,Skinner JR,Schoenfish MJ,Tzekov A,Bensch KG,Bickel PE.Adipocyte protein S3-12 coats nascent lipid droplets.J Biol Chem,2003,278(39):37713~37721
13.Wolins NE,Quaynor BK,Skinner JR,Tzekov A,Croce MA,Gropler MC,Varma V,Yao-Borengasser A,Rasouli N, KernPA,FinckBN,BickelPE.OXPAT/PAT-1isa PPAR-induced lipid droplet protein that promotes fatty acid utilization.Diabetes,2006,55(12):3418~3428
14.Bartz R,Li WH,Venables B,Zehmer JK,Roth MR,Welti R,Anderson RG,Liu P,Chapman KD.Lipidomics reveals thatadiposomesstoreetherlipidsandmediate phospholipid traffic.J Lipid Res,2007,48(4):837~847
15.Goodman JM.The gregarious lipid droplet.J Biol Chem, 2008,283(42):28005~28009
16.Bartz R,Zehmer JK,Liu PS.The new face of lipid droplets.Progress in Biochemistry and Biophysics,2005, 32(5):387~392
17.Mori M,Itabe H,Higashi Y,Fujimoto Y,Shiomi M, Yoshizumi M,Ouchi Y,Takano T.Foam cell formation containing lipid droplets enriched with free cholesterol by hyperlipidemicserum.JLipidRes,2001,42(11): 1771~1781
18.Londos C,Brasaemle DL,Schultz CJ,Adler-Wailes DC, Levin DM,Kimmel AR,Rondinone CM.On the control of lipolysis in adipocytes.Ann N Y Acad Sci,1999,892: 155~168
19.Igal RA,Coleman RA.Neutral lipid storage disease:a geneticdisorderwithabnormalitiesintheregulationof phospholipid metabolism.J Lipid Res,1998,39(1):31~43
20.PattersonMC.Ariddlewrappedinamystery: understanding Niemann-Pick disease,type C.Neurologist, 2003,9(6):301~310
21.Athenstaedt K,Zweytick D,Jandrositz A,Kohlwein SD, Daum G.Identification and characterization of major lipid particle proteins of the yeastSaccharomyces cerevisiae.J Bacteriol,1999,181(20):6441~6448
22.WuCC,HowellKE,NevilleMC,YatesJR3rd, McManaman JL.Proteomics reveal a link between the endoplasmic reticulum and lipid secretory mechanisms in mammary epithelial cells.Electrophoresis,2000,21(16): 3470~3482
23.Umlauf E,Csaszar E,Moertelmaier M,Schuetz GJ,Parton RG,Prohaska R.Association of stomatin with lipid bodies. J Biol Chem,2004,279(22):23699~23709
24.Fujimoto Y,Itabe H,Sakai J,Makita M,Noda J,Mori M, Higashi Y,Kojima S,Takano T.Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7.Biochim Biophys Acta,2004,1644(1):47~59
25.Brasaemle DL,Dolios G,Shapiro L,Wang R.Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes.J Biol Chem, 2004,279(45):46835~46842
26.Kim SC,Chen Y,Mirza S,Xu Y,Lee J,Liu P,Zhao Y.A clean,more efficient method for in-solution digestion of protein mixtures without detergent or urea.J Proteome Res,2006,5(12):3446~3452
27.Binns D,Januszewski T,Chen Y,Hill J,Markin VS,Zhao Y,Gilpin C,Chapman KD,Anderson RG,Goodman JM. An intimate collaboration between peroxisomes and lipid bodies.J Cell Biol,2006,173(5):719~731
28.Beller M,Riedel D,Jansch L,Dieterich G,Wehland J, Jackle H,Kuhnlein RP.Characterization of theDrosophila lipid droplet subproteome.Mol Cell Proteomics,2006,5(6): 1082~1094
29.TurroS,Ingelmo-TorresM,EstanyolJM,TebarF, Fernandez MA,Albor CV,Gaus K,Grewal T,Enrich C, Pol A.Identification and characterization of associated with lipiddropletprotein1:anovelmembrane-associated protein that resides on hepatic lipid droplets.Traffic,2006, 7(9):1254~1269
30.Katavic V,Agrawal GK,Hajduch M,Harris SL,Thelen JJ.Protein and lipid composition analysis of oil bodies from twoBrassica napuscultivars.Proteomics,2006,6(16): 4586~4598
31.Sato S,Fukasawa M,Yamakawa Y,Natsume T,Suzuki T, Shoji I,Aizaki H,Miyamura T,Nishijima M.Proteomic profiling oflipid dropletproteinsinhepatomacelllines expressing hepatitis C virus core protein.J Biochem,2006, 139(5):921~930
32.Cermelli S,Guo Y,Gross SP,Welte MA.The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr Biol,2006,16(18):1783~1795
33.Bartz R,Zehmer JK,Zhu M,Chen Y,Serrero G,Zhao Y, LiuP.Dynamicactivityoflipiddroplets:protein phosphorylation and GTP-mediated protein translocation.J Proteome Res,2007,6(8):3256~3265
34.Wan HC,Melo RC,Jin Z,Dvorak AM,Weller PF.Roles andoriginsofleukocytelipidbodies:proteomicand ultrastructural studies.Faseb J,2007,21(1):167~178
35.Kuerschner L,Moessinger C,Thiele C.Imaging of lipid biosynthesis:howaneutrallipidenterslipiddroplets. Traffic,2008,9(3):338~352
36.Sorger D,Daum G.Synthesis of triacylglycerols by the acyl-coenzyme A:diacyl-glycerol acyltransferase Dga1p in lipid particles of the yeastSaccharomyces cerevisiae.J Bacteriol,2002,184(2):519~524
37.Fujimoto Y,Itabe H,Kinoshita T,Homma KJ,Onoduka J, Mori M,Yamaguchi S,Makita M,Higashi Y,Yamashita A, TakanoT.InvolvementofACSLinlocalsynthesisof neutrallipidsincytoplasmiclipiddropletsinhuman hepatocyte HuH7.J Lipid Res,2007,48(6):1280~1292
38.Haemmerle G,Zimmermann R,Hayn M,Theussl C,Waeg G,Wagner E,Sattler W,Magin TM,Wagner EF,Zechner R.Hormone-sensitivelipasedeficiencyinmicecauses diglyceride accumulation in adipose tissue,muscle,and testis.J Biol Chem,2002,277(7):4806~4815
39.Jenkins CM,Mancuso DJ,Yan W,Sims HF,Gibson B, GrossRW.Identification,cloning,expression,and purificationofthreenovelhumancalcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities.J Biol Chem, 2004,279(47):48968~48975
40.Brasaemle DL,Levin DM,Adler-Wailes DC,Londos C.The lipolyticstimulationof3T3-L1adipocytespromotesthe translocation of hormone-sensitive lipase to the surfaces of lipid storage droplets.Biochim Biophys Acta,2000,1483(2): 251~262
41.Clifford GM,Londos C,Kraemer FB,Vernon RG,Yeaman SJ.Translocation of hormone-sensitive lipase and perilipin upon lipolytic stimulation of rat adipocytes.J Biol Chem, 2000,275(7):5011~5015
42.Zimmermann R,Strauss JG,Haemmerle G,Schoiswohl G, Birner-Gruenberger R,Riederer M,Lass A,Neuberger G, Eisenhaber F,Hermetter A,Zechner R.Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science,2004,306(5700):1383~1386
43.Lass A,Zimmermann R,Haemmerle G,Riederer M, Schoiswohl G,Schweiger M,Kienesberger P,Strauss JG, GorkiewiczG,ZechnerR.Adiposetriglyceridelipasemediatedlipolysisofcellularfatstoresisactivatedby CGI-58 and defective in Chanarin-Dorfman syndrome.Cell Metab,2006,3(5):309~319
44.Lefevre C,Jobard F,Caux F,Bouadjar B,Karaduman A, HeiligR,LakhdarH,WollenbergA,VerretJL, Weissenbach J,Ozguc M,Lathrop M,Prud'homme JF, Fischer J.Mutations in CGI-58,the gene encoding a new proteinoftheesterase/lipase/thioesterasesubfamily,in Chanarin-Dorfman syndrome.Am J Hum Genet,2001, 69(5):1002~1012
45.Huh WK,Falvo JV,Gerke LC,Carroll AS,Howson RW, WeissmanJS,O'SheaEK.Globalanalysisofprotein localization in budding yeast.Nature,2003,425(6959): 686~691
46.Milla P,Athenstaedt K,Viola F,Oliaro-Bosso S,Kohlwein SD,Daum G,Balliano G.Yeast oxidosqualene cyclase (Erg7p)isamajorcomponentoflipidparticles.The Journal of biological chemistry,2002,277(4):2406~2412
47.McGookey DJ,Anderson RG.Morphological characterization ofthecholesterylestercycleinculturedmouse macrophagefoamcells.JCellBiol,1983,97(4): 1156~1168
48.D'Avila H,Melo RC,Parreira GG,Werneck-Barroso E, Castro-Faria-NetoHC,BozzaPT.Mycobacteriumbovis bacillus Calmette-Guerin induces TLR2-mediated formation oflipidbodies:intracellulardomainsforeicosanoid synthesisin vivo.J Immunol,2006,176(5):3087~3097
49.Bozza PT,Yu W,Penrose JF,Morgan ES,Dvorak AM, WellerPF.Eosinophillipidbodies:specific,inducible intracellular sites for enhanced eicosanoid formation.J Exp Med,1997,186(6):909~920
50.Dvorak AM,Morgan E,Schleimer RP,RyeomSW, LichtensteinLM,WellerPF.Ultrastructuralimmunogold localizationofprostaglandinendoperoxidesynthase (cyclooxygenase)to non-membrane-bound cytoplasmic lipid bodies in human lung mast cells,alveolar macrophages, typeIIpneumocytes,andneutrophils.JHistochem Cytochem,1992,40(6):759~769
51.DvorakAM,MorganES,TzizikDM,WellerPF. Prostaglandinendoperoxidesynthase(cyclooxygenase): ultrastructural localization to nonmembrane-bound cytoplasmic lipid bodies in human eosinophils and 3T3 fibroblasts.Int Arch Allergy Immunol,1994,105(3):245~250
52.Weller PF,Monahan-Earley RA,Dvorak HF,Dvorak AM. Cytoplasmic lipid bodies of human eosinophils.Subcellular isolation and analysis of arachidonate incorporation.Am J Pathol,1991,138(1):141~148
53.Bozza PT,Magalhaes KG,Weller PF.Leukocyte lipid bodies——Biogenesisandfunctionsininflammation. Biochim Biophys Acta,2009,1791(6):540~551
54.Meadows JW,Pitzer B,Brockman DE,Myatt L.Expression and localization of adipophilin and perilipin in human fetal membranes:associationwithlipidbodiesandenzymes involvedinprostaglandinsynthesis.JClinEndocrinol Metab,2005,90(4):2344~2350
55.Moreira LS,Piva B,Gentile LB,Mesquita-Santos FP,D'Avila H,Maya-Monteiro CM,Bozza PT,Bandeira-Melo C, DiazBL.CytosolicphospholipaseA(2)-drivenPGE(2) synthesis within unsaturated fatty acids-induced lipid bodies of epithelial cells.Biochim Biophys Acta,2009,1791(3): 156~165
56.Yu W,Bozza PT,Tzizik DM,Gray JP,Cassara J,Dvorak AM,Weller PF.Co-compartmentalization of MAP kinases and cytosolic phospholipase A2 at cytoplasmic arachidonaterich lipid bodies.Am J Pathol,1998,152(3):759~769
57.GuoY,WaltherTC,RaoM,StuurmanN,Goshima G,Terayama K,Wong JS,Vale RD,Walter P,Farese RV.Functional genomic screen reveals genes involved in lipid-droplet formation and utilization.Nature,2008,453 (7195):657~661
58.Kraus-FriedmannN,FengL.Theroleof intracellular Ca2+intheregulationofgluconeogenesis.Metabolism, 1996,45(3):389~403
59.RoulinK,HagensG,HotzR,SauratJH,Veerkamp JH,Siegenthaler G.The fatty acid-binding heterocomplex FA-p34 formed by S100A8 and S100A9 is the major fatty acid carrier in neutrophils and translocates from the cytosol to the membrane upon stimulation.Exp Cell Res,1999, 247(2):410~421
60.van Meer G.Caveolin,cholesterol,andlipid droplets? J Cell Biol,2001,152(5):F29~F34
61.Fujimoto T,Kogo H,Ishiguro K,Tauchi K,Nomura R. Caveolin-2 is targeted to lipid droplets,a new"membrane domain"in the cell.J Cell Biol,2001,152(5):1079~1085
62.OstermeyerAG,Paci JM,ZengY,Lublin DM,Munro S,Brown DA.Accumulation of caveolin in the endoplasmic reticulum redirects the protein to lipid storage droplets.J Cell Biol,2001,152(5):1071~1078
63.Liu P,Rudick M,Anderson RG.Multiple functions of caveolin-1.J Biol Chem,2002,277(44):41295~41298
64.Martin S,Parton RG.Lipid droplets:a unified view of a dynamic organelle.Nat Rev Mol Cell Biol,2006,7(5): 373~378
65.Guo Y,Cordes KR,Farese RV Jr,Walther TC.Lipid droplets at a glance.J Cell Sci,2009,122(Pt 6): 749~752
66.Blanchette-Mackie EJ,Dwyer NK,Barber T,Coxey RA, Takeda T,Rondinone CM,Theodorakis JL,Greenberg AS, Londos C.Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes.J Lipid Res,1995, 36(6):1211~1226
67.Cohen AW,Razani B,Schubert W,Williams TM,Wang XB,Iyengar P,Brasaemle DL,Scherer PE,Lisanti MP. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation.Diabetes,2004,53(5):1261~1270
68.BascomRA,ChanH,RachubinskiRA.Peroxisome biogenesis occurs in an unsynchronized manner in close association with the endoplasmic reticulumintemperaturesensitiveYarrowia lipolyticaPex3p mutants.Mol Biol Cell, 2003,14(3):939~957
Abstract:Lipid droplet is an intracellular structure that consists of a neutral lipid core surrounded by a monolayer of phospholipids.PAT family proteins have been identified as major structural proteins on the surface of lipid droplets.Recently,more than 100 functional proteins have also been found to be associated with lipid droplets by proteomic studies and immunelocalization.These proteins can be categorized into structural proteins,enzymes for lipolysis and synthesis,membrane traffic proteins,and signal transduction proteins.In addition,the lipidomic analysis of animal cell lipid droplets revealed the dynamic activities of the organelle.Therefore,lipid droplets have recently been proposed to be a functional organelle that mediates lipid metabolism,membrane traffic,protein degradation,and signal transduction.Furthermore,recent works demonstrate that prostaglandins are synthesized on lipid droplets in leukocytes and suggest that lipid droplets are able to produce triglyceride and phospholipids.These findings have led to a possibility that lipid droplets not only store lipids for catabolism but also function as an organelle for cellular lipid anabolism.
Key Words:Lipid droplet;Proteomics;Enzymes;Lipid metabolism;Organelle
Lipid Droplet——A Cellular Organelle for Lipid Metabolism
ZHANG Shuyan1,DU Yalan1,2,WANG Yang1,LIU Pingsheng1
1.Institute of Biophysics,Chinese Academy of Sciences,Beijing 100101,China;
2.College of Life Sciences,Guizhou University,Guiyang 550025,China
Q26,Q257
2010-01-29;接受日期:2010-02-02
国家自然科学基金面上项目(09JM241001)
刘平生,电话:(010)64888517,E-mail:pliu@sun5.ibp.ac.cn
This work was supported by a grant from The National Natural Science Foundation of China(09JM241001)
Received:Jan 29,2010Accepted:Feb 2,2010
Corresponding author:LIU Pingsheng,Tel:+86(10)64888517,E-mail:pliu@sun5.ibp.ac.cn
